Design and rationale of the Botswana Smoking Abstinence Reinforcement Trial: a protocol for a stepped-wedge cluster randomized trial
- PMID: 38720363
- PMCID: PMC11077839
- DOI: 10.1186/s43058-024-00588-7
Design and rationale of the Botswana Smoking Abstinence Reinforcement Trial: a protocol for a stepped-wedge cluster randomized trial
Abstract
Background: With expanded and sustained availability of HIV treatment resulting in substantial improvements in life expectancy, the need to address modifiable risk factors associated with leading causes of death among people living with HIV/AIDS (PLWH), such as tobacco smoking, has increased. Tobacco use is highly prevalent among PLWH, especially in southern Africa, where HIV is heavily concentrated, and many people who smoke would like to quit but are unable to do so without assistance. SBIRT (Screening, Brief Intervention and Referral to Treatment) is a well-established evidence-based approach successful at supporting smoking cessation in a variety of settings. Varenicline is efficacious in supporting smoking cessation. We intend to assess the effectiveness of SBIRT and varenicline on smoking cessation among PLWH in Botswana and the effectiveness of our implementation.
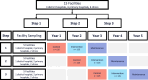
Methods: BSMART (Botswana Smoking Abstinence Reinforcement Trial) is a stepped-wedge, cluster randomized, hybrid Type 2 effectiveness-implementation study guided by the RE-AIM framework, to evaluate the effectiveness and implementation of an SBIRT intervention consisting of the 5As compared to an enhanced standard of care. SBIRT will be delivered by trained lay health workers (LHWs), followed by referral to treatment with varenicline prescribed and monitored by trained nurse prescribers in a network of outpatient HIV care facilities. Seven hundred and fifty people living with HIV who smoke daily and have been receiving HIV care and treatment at one of 15 health facilities will be recruited if they are up to 18 years of age and willing to provide informed consent to participate in the study.
Discussion: BSMART tests a scalable approach to achieve and sustain smoking abstinence implemented in a sustainable way. Integrating an evidence-based approach such as SBIRT, into an HIV care system presents an important opportunity to establish and evaluate a modifiable cancer prevention strategy in a middle-income country (MIC) setting where both LHW and non-physician clinicians are widely used. The findings, including the preliminary cost-effectiveness, will provide evidence to guide the Botswanan government and similar countries as they strive to provide affordable smoking cessation support at scale.
Clinical trial registration: NCT05694637 Registered on 7 December 2022 on clinicaltrials.gov, https://clinicaltrials.gov/search?locStr=Botswana&country=Botswana&cond=Smoking%20Cessation&intr=SBIRT.
Keywords: Brief intervention; Smoking cessation; Type 2 hybrid effectiveness-implementation; Varenicline.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

