Testing novel strategies for patients hospitalised with HIV-associated disseminated tuberculosis (NewStrat-TB): protocol for a randomised controlled trial
- PMID: 38720383
- PMCID: PMC11077808
- DOI: 10.1186/s13063-024-08119-4
Testing novel strategies for patients hospitalised with HIV-associated disseminated tuberculosis (NewStrat-TB): protocol for a randomised controlled trial
Abstract
Background: HIV-associated tuberculosis (TB) contributes disproportionately to global tuberculosis mortality. Patients hospitalised at the time of the diagnosis of HIV-associated disseminated TB are typically severely ill and have a high mortality risk despite initiation of tuberculosis treatment. The objective of the study is to assess the safety and efficacy of both intensified TB treatment (high dose rifampicin plus levofloxacin) and immunomodulation with corticosteroids as interventions to reduce early mortality in hospitalised patients with HIV-associated disseminated TB.
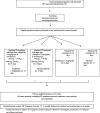
Methods: This is a phase III randomised controlled superiority trial, evaluating two interventions in a 2 × 2 factorial design: (1) high dose rifampicin (35 mg/kg/day) plus levofloxacin added to standard TB treatment for the first 14 days versus standard tuberculosis treatment and (2) adjunctive corticosteroids (prednisone 1.5 mg/kg/day) versus identical placebo for the first 14 days of TB treatment. The study population is HIV-positive patients diagnosed with disseminated TB (defined as being positive by at least one of the following assays: urine Alere LAM, urine Xpert MTB/RIF Ultra or blood Xpert MTB/RIF Ultra) during a hospital admission. The primary endpoint is all-cause mortality at 12 weeks comparing, first, patients receiving intensified TB treatment to standard of care and, second, patients receiving corticosteroids to those receiving placebo. Analysis of the primary endpoint will be by intention to treat. Secondary endpoints include all-cause mortality at 2 and 24 weeks. Safety and tolerability endpoints include hepatoxicity evaluations and corticosteroid-related adverse events.
Discussion: Disseminated TB is characterised by a high mycobacterial load and patients are often critically ill at presentation, with features of sepsis, which carries a high mortality risk. Interventions that reduce this high mycobacterial load or modulate associated immune activation could potentially reduce mortality. If found to be safe and effective, the interventions being evaluated in this trial could be easily implemented in clinical practice.
Trial registration: ClinicalTrials.gov NCT04951986. Registered on 7 July 2021 https://clinicaltrials.gov/study/NCT04951986.
Keywords: Disseminated tuberculosis; HIV; High dose rifampicin; Levofloxacin; Prednisone; Randomised controlled trial.
© 2024. The Author(s).
Conflict of interest statement
There are no competing interests to declare.
Figures
References
-
- Bigna JJR, Noubiap JJN, Agbor AA, Plottel CS, Billong SC, Ayong APR, Koulla-Shiro S. Early mortality during initial treatment of tuberculosis in patients co-infected with HIV at the Yaoundé Central Hospital, Cameroon: an 8-year retrospective cohort study (2006-2013) PLoS One. 2015;10(7):e0132394. doi: 10.1371/journal.pone.0132394. - DOI - PMC - PubMed
-
- Subbarao S, Wilkinson KA, Van Halsema CL, Rao SS, Boyles T, Utay NS, et al. Raised venous lactate and markers of intestinal translocation are associated with mortality among in-patients with HIV-associated TB in rural South Africa. J Acquir Immune Defic Syndr. 2015;70(4):406. doi: 10.1097/QAI.0000000000000763. - DOI - PMC - PubMed
-
- Schutz C, Barr D, Andrade BB, Shey M, Ward A, Janssen S, et al. Clinical, microbiologic, and immunologic determinants of mortality in hospitalized patients with HIV-associated tuberculosis: a prospective cohort study. PLoS Med. 2019;16(7):e1002840. doi: 10.1371/journal.pmed.1002840. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical