Novel role of LLGL2 silencing in autophagy: reversing epithelial-mesenchymal transition in prostate cancer
- PMID: 38720397
- PMCID: PMC11077766
- DOI: 10.1186/s40659-024-00499-w
Novel role of LLGL2 silencing in autophagy: reversing epithelial-mesenchymal transition in prostate cancer
Abstract
Purpose: Prostate cancer (PCa) is a major urological disease that is associated with significant morbidity and mortality in men. LLGL2 is the mammalian homolog of Lgl. It acts as a tumor suppressor in breast and hepatic cancer. However, the role of LLGL2 and the underlying mechanisms in PCa have not yet been elucidated. Here, we investigate the role of LLGL2 in the regulation of epithelial-mesenchymal transition (EMT) in PCa through autophagy in vitro and in vivo.
Methods: PC3 cells were transfected with siLLGL2 or plasmid LLGL2 and autophagy was examined. Invasion, migration, and wound healing were assessed in PC3 cells under autophagy regulation. Tumor growth was evaluated using a shLLGL2 xenograft mouse model.
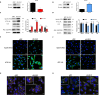
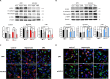
Results: In patients with PCa, LLGL2 levels were higher with defective autophagy and increased EMT. Our results showed that the knockdown of LLGL2 induced autophagy flux by upregulating Vps34 and ATG14L. LLGL2 knockdown inhibits EMT by upregulating E-cadherin and downregulating fibronectin and α-SMA. The pharmacological activation of autophagy by rapamycin suppressed EMT, and these effects were reversed by 3-methyladenine treatment. Interestingly, in a shLLGL2 xenograft mouse model, tumor size and EMT were decreased, which were improved by autophagy induction and worsened by autophagy inhibition.
Conclusion: Defective expression of LLGL2 leads to attenuation of EMT due to the upregulation of autophagy flux in PCa. Our results suggest that LLGL2 is a novel target for alleviating PCa via the regulation of autophagy.
Keywords: Autophagy; EMT; LLGL2; Proliferation; Prostate cancer.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

