The pharmacoepigenetic paradigm in cancer treatment
- PMID: 38720770
- PMCID: PMC11076712
- DOI: 10.3389/fphar.2024.1381168
The pharmacoepigenetic paradigm in cancer treatment
Abstract
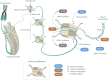
Epigenetic modifications, characterized by changes in gene expression without altering the DNA sequence, play a crucial role in the development and progression of cancer by significantly influencing gene activity and cellular function. This insight has led to the development of a novel class of therapeutic agents, known as epigenetic drugs. These drugs, including histone deacetylase inhibitors, histone acetyltransferase inhibitors, histone methyltransferase inhibitors, and DNA methyltransferase inhibitors, aim to modulate gene expression to curb cancer growth by uniquely altering the epigenetic landscape of cancer cells. Ongoing research and clinical trials are rigorously evaluating the efficacy of these drugs, particularly their ability to improve therapeutic outcomes when used in combination with other treatments. Such combination therapies may more effectively target cancer and potentially overcome the challenge of drug resistance, a significant hurdle in cancer therapy. Additionally, the importance of nutrition, inflammation control, and circadian rhythm regulation in modulating drug responses has been increasingly recognized, highlighting their role as critical modifiers of the epigenetic landscape and thereby influencing the effectiveness of pharmacological interventions and patient outcomes. Epigenetic drugs represent a paradigm shift in cancer treatment, offering targeted therapies that promise a more precise approach to treating a wide spectrum of tumors, potentially with fewer side effects compared to traditional chemotherapy. This progress marks a step towards more personalized and precise interventions, leveraging the unique epigenetic profiles of individual tumors to optimize treatment strategies.
Keywords: DNA methyltransferase inhibitors; clinical trials; epigenetic drugs; histone acetyltransferase inhibitors; histone deacetylase inhibitors; histone methyltransferase inhibitors.
Copyright © 2024 Ocaña-Paredes, Rivera-Orellana, Ramírez-Sánchez, Montalvo-Guerrero, Freire, Espinoza-Ferrao, Altamirano-Colina, Echeverría-Espinoza, Ramos-Medina, Echeverría-Garcés, Granda-Moncayo, Jácome-Alvarado, Andrade and López-Cortés.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

