Biological effects of intraoperative radiation therapy: histopathological changes and immunomodulation in breast cancer patients
- PMID: 38720889
- PMCID: PMC11076837
- DOI: 10.3389/fimmu.2024.1373497
Biological effects of intraoperative radiation therapy: histopathological changes and immunomodulation in breast cancer patients
Abstract
Introduction: Intraoperative radiation therapy (IORT) delivers a single accelerated radiation dose to the breast tumor bed during breast-conserving surgery (BCS). The synergistic biologic effects of simultaneous surgery and radiation remain unclear. This study explores the cellular and molecular changes induced by IORT in the tumor microenvironment and its impact on the immune response modulation.
Methods: Patients with hormone receptor (HR)-positive/HER2-negative, ductal carcinoma in situ (DCIS), or early-stage invasive breast carcinoma undergoing BCS with margin re-excision were included. Histopathological evaluation and RNA-sequencing in the re-excision tissue were compared between patients with IORT (n=11) vs. non-IORT (n=11).
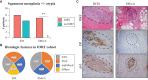
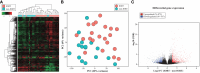
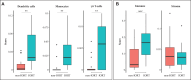
Results: Squamous metaplasia with atypia was exclusively identified in IORT specimens (63.6%, p=0.004), mimicking DCIS. We then identified 1,662 differentially expressed genes (875 upregulated and 787 downregulated) between IORT and non-IORT samples. Gene ontology analyses showed that IORT was associated with the enrichment of several immune response pathways, such as inflammatory response, granulocyte activation, and T-cell activation (p<0.001). When only considering normal tissue from both cohorts, IORT was associated with intrinsic apoptotic signaling, response to gamma radiation, and positive regulation of programmed cell death (p<0.001). Using the xCell algorithm, we inferred a higher abundance of γδ T-cells, dendritic cells, and monocytes in the IORT samples.
Conclusion: IORT induces histological changes, including squamous metaplasia with atypia, and elicits molecular alterations associated with immune response and intrinsic apoptotic pathways. The increased abundance of immune-related components in breast tissue exposed to IORT suggests a potential shift towards active immunogenicity, particularly immune-desert tumors like HR-positive/HER2-negative breast cancer.
Keywords: breast neoplasms; immune response; intraoperative radiation therapy-IORT; squamous metaplasia; tumor microenvironment.
Copyright © 2024 Orozco, Valdez, Matsuba, Simanonok, Ensenyat-Mendez, Ramiscal, Salomon, Takasumi and Grumley.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. (2011) 378:1707–16. doi: 10.1016/S0140-6736(11)61629-2 - DOI - PMC - PubMed
-
- Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. (2014) 383:603–13. doi: 10.1016/S0140-6736(13)61950-9 - DOI - PubMed
-
- Vaidya JS, Bulsara M, Baum M, Wenz F, Massarut S, Pigorsch S, et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ. (2020) 370:m2836. doi: 10.1136/bmj.m2836 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

