Benzo[ a][1,4]benzothia-zino[3,2- c]phenothia-zine
- PMID: 38721004
- PMCID: PMC11074542
- DOI: 10.1107/S2414314624003572
Benzo[ a][1,4]benzothia-zino[3,2- c]phenothia-zine
Abstract
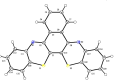
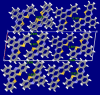
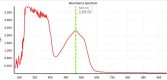
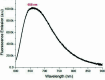
The title compound, C22H12N2S2, crystallizes in space group P21/c with four mol-ecules in the asymmetric unit. The heterocyclic mol-ecule is quasi-planar with a dihedral angle between the phenyl rings on the periphery of the mol-ecule of 1.73 (19)°. Short H⋯S (2.92 Å) and C-H⋯π [2.836 (3) Å] contacts are observed in the crystal with shorted π-π stacking distances of 3.438 (3) Å along the b axis. Surprisingly, and unlike a closely related material, this mol-ecule readily forms large crystals by sublimation and by slow evaporation from di-chloro-methane. The maximum absorbance in the UV-Vis spectrum is at 533 nm. Emission was measured upon excitation at 533 nm with a fluorescence λmax of 658 nm and cutoff of 900 nm.
Keywords: crystal structure; donor/acceptor; dyes; fused heterocyclic aromatics; ladder oligomers; organic semiconductors.
© Bader et al. 2024.
Figures
Similar articles
-
13-Nitro-benzo[a][1,4]benzo-thia-zino[3,2-c]phenoxazine.IUCrdata. 2024 Apr 26;9(Pt 4):x240299. doi: 10.1107/S2414314624002992. eCollection 2024 Apr. IUCrdata. 2024. PMID: 38720996 Free PMC article.
-
4-[(1E)-({[(Benzyl-sulfan-yl)methane-thio-yl]amino}-imino)-meth-yl]benzene-1,3-diol chloro-form hemisolvate: crystal structure, Hirshfeld surface analysis and computational study.Acta Crystallogr E Crystallogr Commun. 2020 Jun 2;76(Pt 7):990-997. doi: 10.1107/S2056989020007070. eCollection 2020 Jul 1. Acta Crystallogr E Crystallogr Commun. 2020. PMID: 32695439 Free PMC article.
-
Crystal structure of (Z)-2-(1-benzyl-2-oxoindolin-3-yl-idene)-N-phenyl-hydra-zine-1-carbo-thio-amide.Acta Crystallogr E Crystallogr Commun. 2015 Feb 7;71(Pt 3):o160-1. doi: 10.1107/S2056989015002248. eCollection 2015 Mar 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 25844226 Free PMC article.
-
5-Chloro-N-(4,5-dihydro-1H-imidazol-2-yl)-2,1,3-benzothia-diazol-4-amine (tizanidine).Acta Crystallogr Sect E Struct Rep Online. 2011 Apr 1;67(Pt 4):o838-9. doi: 10.1107/S1600536811008348. Epub 2011 Mar 12. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 21754121 Free PMC article.
-
1-{4-Chloro-2-[2-(2-fluoro-phen-yl)-1,3-dithio-lan-2-yl]phen-yl}-2-methyl-1H-imidazole-5-carbaldehyde.Acta Crystallogr Sect E Struct Rep Online. 2011 Jan 26;67(Pt 2):o496-7. doi: 10.1107/S1600536811002595. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 21523151 Free PMC article.
References
-
- Agarwal, N. L. & Schaefer, W. (1980). J. Org. Chem. 45, 2155–2161.
-
- Bruker (2014). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Feister, C., Pham, P.-T. T. & Bradley, A. (2023). MRS Advances, pp. 889–893. https://doi.org/10.1557/s43580-023-00609-y
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous