Efficacy of COVID-19 Vaccines in Patients with Hematological Malignancy Compared to Healthy Controls: A Systematic Review and Meta-analysis
- PMID: 38721084
- PMCID: PMC11074501
- DOI: 10.31662/jmaj.2023-0171
Efficacy of COVID-19 Vaccines in Patients with Hematological Malignancy Compared to Healthy Controls: A Systematic Review and Meta-analysis
Abstract
Background: The possibility of developing a severe coronavirus infectious (COVID-19) disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has increased, particularly in patients with hematological malignancies. These patients are more likely to produce less antibody protection due to the immunocompromised nature of the disease and the anticancer treatments. Therefore, the present systematic review intended to evaluate the seroconversion rate of COVID-19 vaccines in patients with hematological malignancies compared with healthy controls.
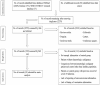
Methods: A comprehensive systematic search was conducted in Medline via PubMed, EMBASE, and the World Health Organization COVID-19 Research Database, as well as other searches (i.e., reference list from article search and manual searches), from December 2020 to May 2022. The outcome of interest included estimating the seroconversion rates following COVID-19 vaccination in patients with hematological malignancies and comparing them with those in healthy controls. After two-step screening, the data were extracted and the summary measures were calculated using a random-effects model.
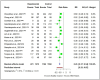
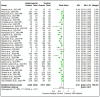
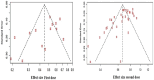
Results: A total of 39 articles regarding patients with hematological malignancies were included in the present review. After the first vaccine dose, these patients had considerably lower antibody response rates (37.0%) compared with healthy controls (74.5%). Following the second vaccine dose, the seroconversion rate in patients reached 66.8%, whereas it peaked at 97.9% in the healthy controls following complete immunization. Notably, the BNT162b2 and ChAdOx1 vaccine combination achieved the highest seropositivity rate of approximately 70%. Multiple myeloma, chronic lymphocytic leukemia, and lymphoma were the cancers of interest in most of the studies.
Conclusions: The results of the present study highlighted the comparatively low seropositivity rates in patients with hematological malignancies, with substantial variations in rates across disease groups. The findings emphasize the possibility of additional booster doses for these individuals to enhance their immunity against SARS-CoV-2.
Keywords: COVID-19 vaccines; Hematological malignancy; Immunogenicity; Seroconversion; Systematic review.
Copyright © Japan Medical Association.
Conflict of interest statement
None
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
