Impact of the COVID-19 pandemic on routine surveillance for adults with chronic hepatitis B virus (HBV) infection in the UK
- PMID: 38721280
- PMCID: PMC11077619
- DOI: 10.12688/wellcomeopenres.17522.2
Impact of the COVID-19 pandemic on routine surveillance for adults with chronic hepatitis B virus (HBV) infection in the UK
Abstract
Background: To determine the impact of the COVID-19 pandemic on the population with chronic Hepatitis B virus (HBV) infection under hospital follow-up in the UK, we quantified the coverage and frequency of measurements of biomarkers used for routine surveillance (alanine transferase [ALT] and HBV viral load).
Methods: We used anonymized electronic health record data from the National Institute for Health Research (NIHR) Health Informatics Collaborative (HIC) pipeline representing five UK National Health Service (NHS) Trusts.
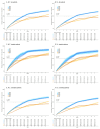
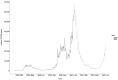
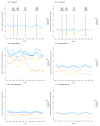
Results: We report significant reductions in surveillance of both biomarkers during the pandemic compared to pre-COVID-19 years, both in terms of the proportion of patients who had ≥1 measurement annually, and the mean number of measurements per patient.
Conclusions: These results demonstrate the real-time utility of HIC data in monitoring health-care provision, and support interventions to provide catch-up services to minimise the impact of the pandemic. Further investigation is required to determine whether these disruptions will be associated with increased rates of adverse chronic HBV outcomes.
Keywords: COVID-19; HBV; epidemiology; hepatitis B virus; viral hepatitis; virology.
Copyright: © 2023 Campbell C et al.
Conflict of interest statement
Competing interests: GC reports personal fees from Gilead and Merck Sharp & Dohme outside the submitted work. BG reports other from Imperial National Institute for Health Research (NIHR) Biomedical Research Centres (BRC), during the conduct of the study. EN reports grants from ViiV healthcare, grants from GlaxoSmithKline (GSK), grants from Gilead, outside the submitted work. Other authors have no conflict of interest.
Figures
References
-
- Global Health Sector Strategy on viral hepatitis 2016– 2021.Geneva;2016; [cited 2021 Jun 14]. Reference Source
-
- European Association for the Study of the Liver: The summarized of EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98. - PubMed
LinkOut - more resources
Full Text Sources
Medical

