Genetic basis of local adaptation in the cold-tolerant mangrove Kandelia obovata
- PMID: 38721336
- PMCID: PMC11076828
- DOI: 10.3389/fpls.2024.1385210
Genetic basis of local adaptation in the cold-tolerant mangrove Kandelia obovata
Abstract
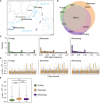
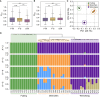
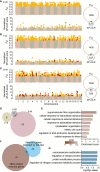
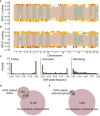
Understanding the genetic basis of local adaption is crucial in the context of global climate change. Mangroves, as salt-tolerant trees and shrubs in the intertidal zone of tropical and subtropical coastlines, are particularly vulnerable to climate change. Kandelia obovata, the most cold-tolerant mangrove species, has undergone ecological speciation from its cold-intolerant counterpart, Kandelia candel, with geographic separation by the South China Sea. In this study, we conducted whole-genome re-sequencing of K. obovata populations along China's southeast coast, to elucidate the genetic basis responsible for mangrove local adaptation to climate. Our analysis revealed a strong population structure among the three K. obovata populations, with complex demographic histories involving population expansion, bottleneck, and gene flow. Genome-wide scans unveiled pronounced patterns of selective sweeps in highly differentiated regions among pairwise populations, with stronger signatures observed in the northern populations compared to the southern population. Additionally, significant genotype-environment associations for temperature-related variables were identified, while no associations were detected for precipitation. A set of 39 high-confidence candidate genes underlying local adaptation of K. obovata were identified, which are distinct from genes under selection detected by comparison between K. obovata and its cold-intolerant relative K. candel. These results significantly contribute to our understanding of the genetic underpinnings of local adaptation in K. obovata and provide valuable insights into the evolutionary processes shaping the genetic diversity of mangrove populations in response to climate change.
Keywords: demographic history; genome-environment association; local adaptation; mangroves; population genomics; selective sweeps.
Copyright © 2024 Zou, Wang, Zhou and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Physiological and transcriptomic responses to cold waves of the most cold-tolerant mangrove, Kandelia obovata.Front Plant Sci. 2023 Feb 10;14:1069055. doi: 10.3389/fpls.2023.1069055. eCollection 2023. Front Plant Sci. 2023. PMID: 36844068 Free PMC article.
-
Phenotypic adaptation and genomic variation of Kandelia obovata associated with its northern introduction along southeastern coast of China.Front Plant Sci. 2025 Mar 26;16:1512620. doi: 10.3389/fpls.2025.1512620. eCollection 2025. Front Plant Sci. 2025. PMID: 40206876 Free PMC article.
-
A comparison of 25 complete chloroplast genomes between sister mangrove species Kandelia obovata and Kandelia candel geographically separated by the South China Sea.Front Plant Sci. 2023 Jan 4;13:1075353. doi: 10.3389/fpls.2022.1075353. eCollection 2022. Front Plant Sci. 2023. PMID: 36684775 Free PMC article.
-
[Advances in molecular biological studies of the mangrove Kandelia obovata Sheue, Liu & Yong].Sheng Wu Gong Cheng Xue Bao. 2017 Feb 25;33(2):196-204. doi: 10.13345/j.cjb.160272. Sheng Wu Gong Cheng Xue Bao. 2017. PMID: 28956376 Review. Chinese.
-
Genome-Wide Identification, Characterization, and Expression Analysis of the Copper-Containing Amine Oxidase Gene Family in Mangrove Kandelia obovata.Int J Mol Sci. 2023 Dec 9;24(24):17312. doi: 10.3390/ijms242417312. Int J Mol Sci. 2023. PMID: 38139139 Free PMC article. Review.
Cited by
-
Thermal sensitivity and niche plasticity of generalist and specialist leaf-endophytic bacteria in Mangrove Kandelia obovata.Commun Biol. 2025 Jan 3;8(1):5. doi: 10.1038/s42003-024-07446-1. Commun Biol. 2025. PMID: 39753754 Free PMC article.
References
-
- Angert A. L., Bontrager M. G., Aringgren J. (2020). What Do We Really Know about Adaptation at Range Edges? Annu. Rev. Ecol. Evol. Syst. 51, 341–361. doi: 10.1146/annurev-ecolsys-012120-091002 - DOI
LinkOut - more resources
Full Text Sources

