Cilia-deficient renal tubule cells are primed for injury with mitochondrial defects and aberrant tryptophan metabolism
- PMID: 38721661
- PMCID: PMC11390130
- DOI: 10.1152/ajprenal.00225.2023
Cilia-deficient renal tubule cells are primed for injury with mitochondrial defects and aberrant tryptophan metabolism
Abstract
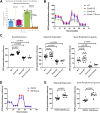
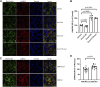
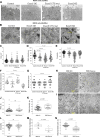
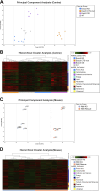
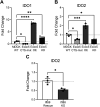
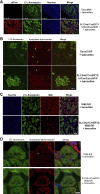
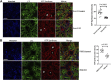
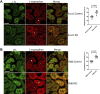
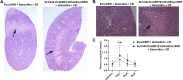
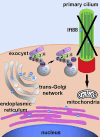
The exocyst and Ift88 are necessary for primary ciliogenesis. Overexpression of Exoc5 (OE), a central exocyst component, resulted in longer cilia and enhanced injury recovery. Mitochondria are involved in acute kidney injury (AKI). To investigate cilia and mitochondria, basal respiration and mitochondrial maximal and spare respiratory capacity were measured in Exoc5 OE, Exoc5 knockdown (KD), Exoc5 ciliary targeting sequence mutant (CTS-mut), control Madin-Darby canine kidney (MDCK), Ift88 knockout (KO), and Ift88 rescue cells. In Exoc5 KD, Exoc5 CTS-mut, and Ift88 KO cells, these parameters were decreased. In Exoc5 OE and Ift88 rescue cells they were increased. Reactive oxygen species were higher in Exoc5 KD, Exoc5 CTS-mut, and Ift88 KO cells compared with Exoc5 OE, control, and Ift88 rescue cells. By electron microscopy, mitochondria appeared abnormal in Exoc5 KD, Exoc5 CTS-mut, and Ift88 KO cells. A metabolomics screen of control, Exoc5 KD, Exoc5 CTS-mut, Exoc5 OE, Ift88 KO, and Ift88 rescue cells showed a marked increase in tryptophan levels in Exoc5 CTS-mut (113-fold) and Exoc5 KD (58-fold) compared with control cells. A 21% increase was seen in Ift88 KO compared with rescue cells. In Exoc5 OE compared with control cells, tryptophan was decreased 59%. To determine the effects of ciliary loss on AKI, we generated proximal tubule-specific Exoc5 and Ift88 KO mice. These mice had loss of primary cilia, decreased mitochondrial ATP synthase, and increased tryptophan in proximal tubules with greater injury following ischemia-reperfusion. These data indicate that cilia-deficient renal tubule cells are primed for injury with mitochondrial defects in tryptophan metabolism.NEW & NOTEWORTHY Mitochondria are centrally involved in acute kidney injury (AKI). Here, we show that cilia-deficient renal tubule cells both in vitro in cell culture and in vivo in mice are primed for injury with mitochondrial defects and aberrant tryptophan metabolism. These data suggest therapeutic strategies such as enhancing ciliogenesis or improving mitochondrial function to protect patients at risk for AKI.
Keywords: acute kidney injury; exocyst; mitochondria; oxidative stress; primary cilia.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Lobo GP, Fulmer D, Guo L, Zuo X, Dang Y, Kim SH, Su Y, George K, Obert E, Fogelgren B, Nihalani D, Norris RA, Rohrer B, Lipschutz JH. The exocyst is required for photoreceptor ciliogenesis and retinal development. J Biol Chem 292: 14814–14826, 2017. doi: 10.1074/jbc.M117.795674. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

