SIRT3 protects endometrial receptivity in patients with polycystic ovary syndrome
- PMID: 38721809
- PMCID: PMC12091677
- DOI: 10.1097/CM9.0000000000003127
SIRT3 protects endometrial receptivity in patients with polycystic ovary syndrome
Abstract
Background: The sirtuin family is well recognized for its crucial involvement in various cellular processes. Nevertheless, studies on its role in the human endometrium are limited. This study aimed to explore the expression and localization of the sirtuin family in the human endometrium, focusing on sirtuin 3 (SIRT3) and its potential role in the oxidative imbalance of the endometrium in polycystic ovary syndrome (PCOS).
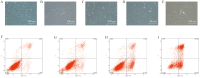
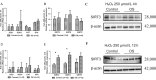
Methods: Endometrial specimens were collected from both patients with PCOS and controls undergoing hysteroscopy at the Center for Reproductive Medicine, Peking University Third Hospital, from July to August 2015 and used for cell culture. The protective effects of SIRT3 were investigated, and the mechanism of SIRT3 in improving endometrial receptivity of patients with PCOS was determined using various techniques, including cellular bioenergetic analysis, small interfering ribonucleic acid (siRNA) silencing, real-time quantitative polymerase chain reaction, Western blot, immunofluorescence, immunohistochemistry, and flow cytometry analysis.
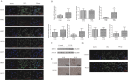
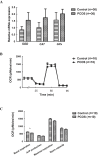
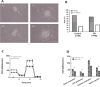
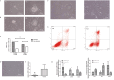
Results: The sirtuin family was widely expressed in the human endometrium, with SIRT3 showing a significant increase in expression in patients with PCOS compared with controls ( P <0.05), as confirmed by protein and gene assays. Concurrently, endometrial antioxidant levels were elevated, while mitochondrial respiratory capacity was reduced, in patients with PCOS ( P <0.05). An endometrial oxidative stress (OS) model revealed that the downregulation of SIRT3 impaired the growth and proliferation status of endometrial cells and reduced their receptivity to day 4 mouse embryos. The results suggested that SIRT3 might be crucial in maintaining normal cellular state by regulating antioxidants, cell proliferation, and apoptosis, thereby contributing to enhanced endometrial receptivity.
Conclusions: Our findings proposed a significant role of SIRT3 in improving endometrial receptivity in patients with PCOS by alleviating OS and regulating the balance between cell proliferation and apoptosis. Therefore, SIRT3 could be a promising target for predicting and improving endometrial receptivity in this patient population.
Keywords: Apoptosis; Endometrial receptivity; Oxidative stress; Polycystic ovary syndrome; Sirtuin 3.
Copyright © 2024 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
None.
Figures
Similar articles
-
Berberine Protects Against Dihydrotestosterone-Induced Human Ovarian Granulosa Cell Injury and Ferroptosis by Regulating the Circ_0097636/MiR-186-5p/SIRT3 Pathway.Appl Biochem Biotechnol. 2024 Aug;196(8):5265-5282. doi: 10.1007/s12010-023-04825-y. Epub 2023 Dec 28. Appl Biochem Biotechnol. 2024. PMID: 38153651
-
Mitochondrial and glucose metabolic dysfunctions in granulosa cells induce impaired oocytes of polycystic ovary syndrome through Sirtuin 3.Free Radic Biol Med. 2022 Jul;187:1-16. doi: 10.1016/j.freeradbiomed.2022.05.010. Epub 2022 May 17. Free Radic Biol Med. 2022. PMID: 35594990
-
Correlation between endometrial receptivity with expressions of IL-1 and VEGF in rats with polycystic ovary syndrome.Eur Rev Med Pharmacol Sci. 2019 Jul;23(13):5575-5580. doi: 10.26355/eurrev_201907_18291. Eur Rev Med Pharmacol Sci. 2019. PMID: 31298309
-
An Update on the Progress of Endometrial Receptivity in Women with Polycystic Ovary Syndrome.Reprod Sci. 2022 Aug;29(8):2136-2144. doi: 10.1007/s43032-021-00641-z. Epub 2021 Jun 2. Reprod Sci. 2022. PMID: 34076874 Review.
-
The Disorders of Endometrial Receptivity in PCOS and Its Mechanisms.Reprod Sci. 2022 Sep;29(9):2465-2476. doi: 10.1007/s43032-021-00629-9. Epub 2021 May 27. Reprod Sci. 2022. PMID: 34046867 Review.
Cited by
-
Higher oxidative balance score is associated with lower female infertility: a cross-sectional study.Front Nutr. 2024 Dec 4;11:1484756. doi: 10.3389/fnut.2024.1484756. eCollection 2024. Front Nutr. 2024. PMID: 39703331 Free PMC article.
-
Managing uterine artery pseudoaneurysm post-hysteroscopic surgery: Clinical insights and future directions.World J Clin Cases. 2024 Nov 16;12(32):6547-6550. doi: 10.12998/wjcc.v12.i32.6547. World J Clin Cases. 2024. PMID: 39554891 Free PMC article.
References
-
- Ashkenazi J Farhi J Orvieto R Homburg R Dekel A Feldberg D, et al. . Polycystic ovary syndrome patients as oocyte donors: The effect of ovarian stimulation protocol on the implantation rate of the recipient. Fertil Steril 1995;64:564–567. doi: 10.1016/s0015-0282(16)57793-0. - PubMed
-
- Fiorentino G Cimadomo D Innocenti F Soscia D Vaiarelli A Ubaldi FM, et al. . Biomechanical forces and signals operating in the ovary during folliculogenesis and their dysregulation: Implications for fertility. Hum Reprod Update 2023;29:1–23. doi: 10.1093/humupd/dmac031. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

