Synthesis of Stereodefined Polysubstituted Bicyclo[1.1.0]butanes
- PMID: 38722207
- PMCID: PMC11117409
- DOI: 10.1021/jacs.4c04438
Synthesis of Stereodefined Polysubstituted Bicyclo[1.1.0]butanes
Erratum in
-
Addition to "Synthesis of Stereodefined Polysubstituted Bicyclo[1.1.0]butanes".J Am Chem Soc. 2024 Jun 26;146(25):17530. doi: 10.1021/jacs.4c06987. Epub 2024 Jun 13. J Am Chem Soc. 2024. PMID: 38868983 Free PMC article. No abstract available.
Abstract
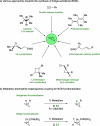
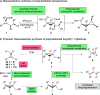
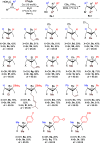
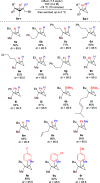
We report a highly diastereoselective synthesis of polysubstituted bicyclobutanes possessing up to three stereodefined quaternary centers and five substituents. Our strategy involves a diastereoselective carbometalation of cyclopropenes followed by a cyclization to furnish the bicyclobutane ring system. This straightforward approach allows for the incorporation of a diverse range of substituents and functional groups, notably without the need for electron-withdrawing functionalities.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
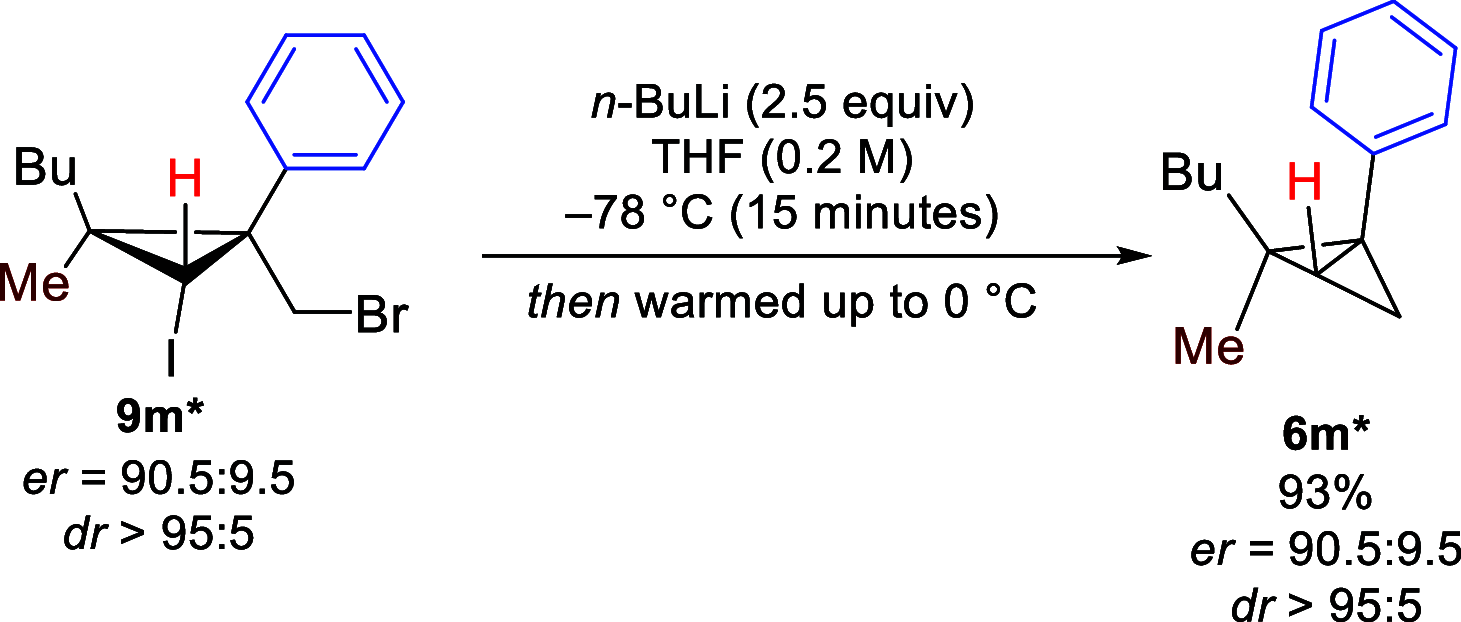
References
-
- Wiberg K. B. The Concept of Strain in Organic Chemistry. Angew. Chem., Int. Ed. Engl. 1986, 25, 312–322. 10.1002/anie.198603121. - DOI
-
- Wiberg K. B.; Ciula P. R. ETHYL BICYCLO[1.1.0]BUTANE–1–CARBOXYLATE. J. Am. Chem. Soc. 1959, 81, 5261–5262. 10.1021/ja01528a060. - DOI
-
- Wiberg K. B.; Lampman G. M.; Ciula R. P.; Connor D. S.; Schertler P.; Lavanish J. Bicyclo[1.1.0]butane. Tetrahedron 1965, 21, 2749–2769. 10.1016/S0040-4020(01)98361-9. - DOI
-
- Hoz S.Bicyclo [1.1.0]butane. In The Chemistry of the Cyclopropyl Group; Rappoport Z., Ed.; John Wiley & Sons, 1987; pp 1121–1192.
LinkOut - more resources
Full Text Sources

