HERC3 facilitates ERAD of select membrane proteins by recognizing membrane-spanning domains
- PMID: 38722278
- PMCID: PMC11082371
- DOI: 10.1083/jcb.202308003
HERC3 facilitates ERAD of select membrane proteins by recognizing membrane-spanning domains
Abstract
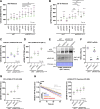
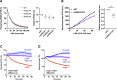
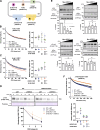
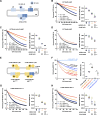
Aberrant proteins located in the endoplasmic reticulum (ER) undergo rapid ubiquitination by multiple ubiquitin (Ub) E3 ligases and are retrotranslocated to the cytosol as part of the ER-associated degradation (ERAD). Despite several ERAD branches involving different Ub E3 ligases, the molecular machinery responsible for these ERAD branches in mammalian cells remains not fully understood. Through a series of multiplex knockdown/knockout experiments with real-time kinetic measurements, we demonstrate that HERC3 operates independently of the ER-embedded ubiquitin ligases RNF5 and RNF185 (RNF5/185) to mediate the retrotranslocation and ERAD of misfolded CFTR. While RNF5/185 participates in the ERAD process of both misfolded ABCB1 and CFTR, HERC3 uniquely promotes CFTR ERAD. In vitro assay revealed that HERC3 directly interacts with the exposed membrane-spanning domains (MSDs) of CFTR but not with the MSDs embedded in liposomes. Therefore, HERC3 could play a role in the quality control of MSDs in the cytoplasm and might be crucial for the ERAD pathway of select membrane proteins.
© 2024 Kamada et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures









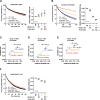
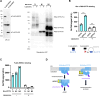
Comment in
-
The HERCulean task of recognizing, ubiquitinating, and shielding misfolded integral membrane proteins.J Cell Biol. 2024 Jul 1;223(7):e202405160. doi: 10.1083/jcb.202405160. Epub 2024 Jun 5. J Cell Biol. 2024. PMID: 38836811 Free PMC article.
References
-
- Capurro, V., Tomati V., Sondo E., Renda M., Borrelli A., Pastorino C., Guidone D., Venturini A., Giraudo A., Mandrup Bertozzi S., et al. . 2021. Partial rescue of F508del-CFTR stability and trafficking defects by double corrector treatment. Int. J. Mol. Sci. 22:5262. 10.3390/ijms22105262 - DOI - PMC - PubMed
-
- Chen, B., Mariano J., Tsai Y.C., Chan A.H., Cohen M., and Weissman A.M.. 2006. The activity of a human endoplasmic reticulum-associated degradation E3, gp78, requires its Cue domain, RING finger, and an E2-binding site. Proc. Natl. Acad. Sci. USA. 103:341–346. 10.1073/pnas.0506618103 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

