Prolonged depletion of profilin 1 or F-actin causes an adaptive response in microtubules
- PMID: 38722279
- PMCID: PMC11082369
- DOI: 10.1083/jcb.202309097
Prolonged depletion of profilin 1 or F-actin causes an adaptive response in microtubules
Abstract
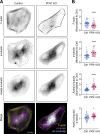
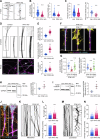
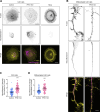
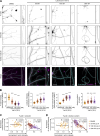
In addition to its well-established role in actin assembly, profilin 1 (PFN1) has been shown to bind to tubulin and alter microtubule growth. However, whether PFN1's predominant control over microtubules in cells occurs through direct regulation of tubulin or indirectly through the polymerization of actin has yet to be determined. Here, we manipulated PFN1 expression, actin filament assembly, and actomyosin contractility and showed that reducing any of these parameters for extended periods of time caused an adaptive response in the microtubule cytoskeleton, with the effect being significantly more pronounced in neuronal processes. All the observed changes to microtubules were reversible if actomyosin was restored, arguing that PFN1's regulation of microtubules occurs principally through actin. Moreover, the cytoskeletal modifications resulting from PFN1 depletion in neuronal processes affected microtubule-based transport and mimicked phenotypes that are linked to neurodegenerative disease. This demonstrates how defects in actin can cause compensatory responses in other cytoskeleton components, which in turn significantly alter cellular function.
© 2024 Cisterna et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures





Update of
-
Cytoskeletal adaptation following long-term dysregulation of actomyosin in neuronal processes.bioRxiv [Preprint]. 2023 Sep 10:2023.08.25.554891. doi: 10.1101/2023.08.25.554891. bioRxiv. 2023. Update in: J Cell Biol. 2024 Jul 1;223(7):e202309097. doi: 10.1083/jcb.202309097. PMID: 37662186 Free PMC article. Updated. Preprint.
Comment in
-
Profilin affects microtubule dynamics via actin.J Cell Biol. 2024 Jul 1;223(7):e202404112. doi: 10.1083/jcb.202404112. Epub 2024 Jun 4. J Cell Biol. 2024. PMID: 38832903 Free PMC article.
References
-
- Bender, M., Stritt S., Nurden P., van Eeuwijk J.M., Zieger B., Kentouche K., Schulze H., Morbach H., Stegner D., Heinze K.G., et al. . 2014. Megakaryocyte-specific Profilin1-deficiency alters microtubule stability and causes a Wiskott-Aldrich syndrome-like platelet defect. Nat. Commun. 5:4746. 10.1038/ncomms5746 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

