Network Meta-analysis of Ravulizumab and Alternative Interventions for the Treatment of Neuromyelitis Optica Spectrum Disorder
- PMID: 38722571
- PMCID: PMC11136926
- DOI: 10.1007/s40120-024-00597-7
Network Meta-analysis of Ravulizumab and Alternative Interventions for the Treatment of Neuromyelitis Optica Spectrum Disorder
Erratum in
-
Correction: Network Meta-analysis of Ravulizumab and Alternative Interventions for the Treatment of Neuromyelitis Optica Spectrum Disorder.Neurol Ther. 2024 Aug;13(4):1313-1314. doi: 10.1007/s40120-024-00638-1. Neurol Ther. 2024. PMID: 38874709 Free PMC article. No abstract available.
Abstract
Introduction: Anti-aquaporin-4 antibody-positive (AQP4-Ab+) neuromyelitis optica spectrum disorder (NMOSD) is a complement-mediated autoimmune disease in which unpredictable and relapsing attacks on the central nervous system cause irreversible and accumulating damage. Comparative efficacy of new NMOSD therapies, such as ravulizumab, with established therapies is critical in making informed treatment decisions.
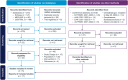
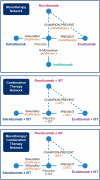
Methods: Efficacy of ravulizumab relative to established AQP4-Ab+ NMOSD treatments, such as eculizumab, inebilizumab, and satralizumab, was evaluated in a Bayesian network meta-analysis (NMA). Data were extracted from trials identified by a systematic literature review. The final evidence base consisted of 17 publications representing five unique and global studies (PREVENT, N-MOmentum, SAkuraSky, SAkuraStar, and CHAMPION-NMOSD). The primary endpoint was time-to-first relapse; other outcomes included annualized relapse rates (ARRs).
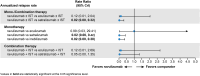
Results: For patients receiving monotherapy (monoclonal antibody only), ravulizumab was associated with a lower risk of relapse than inebilizumab (hazard ratio [HR] 0.09, 95% credible interval [CrI] 0.02, 0.57) or satralizumab (HR 0.08, 95% CrI 0.01, 0.55) and was comparable to eculizumab (HR 0.86, 95% Crl 0.16, 4.52). Ravulizumab + immunosuppressive therapy (IST) was associated with a lower risk of relapse than satralizumab + IST (HR 0.15, 95% CrI 0.03, 0.78); the comparison with eculizumab + IST suggested no difference. No patients treated with inebilizumab received background IST and were thus excluded from analysis. The ARR with ravulizumab monotherapy was 98% lower compared with inebilizumab (rate ratio [RR] 0.02, 95% Crl 0.00, 0.38) and satralizumab (RR 0.02, 95% Crl 0.00, 0.42) monotherapies. The ARR with ravulizumab ± IST showed the strongest treatment-effect estimates compared with other interventions.
Conclusion: In the absence of head-to-head randomized controlled trials, NMA results suggest ravulizumab, a C5 inhibitor, is likely to be more effective in preventing NMOSD relapse in patients with AQP4-Ab+ NMOSD when compared with other treatments having different methods of action.
Keywords: Aquaporin-4; Eculizumab; Inebilizumab; Network meta-analysis; Neuromyelitis optica; Ravulizumab; Satralizumab.
Plain language summary
Anti-aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder, also called AQP4-Ab+ NMOSD, is a rare autoimmune disease that causes repeated episodes of symptoms such as blindness, arm/leg weakness, painful spasms, vomiting, and hiccups, among other symptoms. Each episode can cause nervous system damage to worsen, making it more difficult to recover back to regular abilities. Repeated episodes are likely to cause permanent damage, such as blindness and paralysis. Medical treatments that reduce episodes also reduce the damage and the chances symptoms will become permanent. One treatment, ravulizumab, is being studied to treat adults with AQP4-Ab+ NMOSD. This analysis looked at information from published clinical studies to compare ravulizumab with three other treatments (eculizumab, inebilizumab, and satralizumab) to determine how well each treatment reduced NMOSD episodes. There are no studies that have tested all four treatments in one study. Here, the treatments were compared by a method used to estimate the likelihood of a treatment being better than the others. While all four treatments successfully reduced episodes in their own studies, this analysis predicts that ravulizumab would likely be best in preventing episodes compared with inebilizumab or satralizumab when used alone or in combination with other immunosuppressive treatments. These findings, in consideration along with other relevant factors such as cost, safety, dosing delivery method, and frequency of treatment, may help doctors and patients decide what is the best treatment option for each individual patient to prevent attacks in adults with AQP4-Ab+ NMOSD.
© 2024. The Author(s).
Conflict of interest statement
Stacey L. Clardy is an employee of the University of Utah and the Salt Lake City VA. She and her institution have received research support from NIH/NINDS (U01, The ExTINGUISH Trial), the Western Institute for Veteran Research, the Siegel Rare Neuroimmune Association, the Immune Deficiency Foundation, Viela Bio/Horizon, Alexion/AstraZeneca, the Barbara Gural Steinmetz Foundation, and the Sumaira Foundation for NMO, and consulting/advisory board fees from Alexion, VielaBio/Horizon, and Genentech/Roche. Sean J. Pittock has received personal compensation for serving on scientific advisory boards for F. Hoffmann-La Roche (which manufactures satralizumab, an approved targeted therapy for NMOSD) and his institution has received grants; his institution has received grants, personal fees, nonfinancial support, research support, and compensation for serving as a consultant for Alexion, AstraZeneca Rare Disease (which holds the patent rights to ravulizumab and eculizumab). He holds Patent # 9,891,219B2, Application # 12–573,942, Methods for Treating Neuromyelitis Optica (NMO) by Administration of Eculizumab to an Individual That Is Aquaporin-4 (AQP4)-IgG Autoantibody Positive, which has been issued and for which he has received royalties. He also reports grants, personal fees, nonfinancial support, and other support from Horizon, MedImmune (which produces inebilizumab). Orhan Aktas reports grants from the German Ministry of Education and Research (BMBF) and the German Research Foundation (DFG); grants and personal fees from Biogen, Genzyme, Novartis, and Teva, and personal fees from Alexion, Almirall, Bristol Myers Squibb, Horizon Therapeutics, Merck Serono, and Roche, and is a member of the European Reference Network for Rare Eye Diseases (ERN-EYE), co-funded by the Health Program of the European Union under the Framework Partnership Agreement No 739434 ‘ERN-EYE’. Jin Nakahara reports personal fees from AbbVie, Alexion Pharma GK, Asahi Kasei Medical, Biogen, Bristol Myers Squibb, Chugai, CSL Behring, Daiichi Sankyo, Eisai, Kyorin, Mitsubishi Tanabe Pharma, Novartis, Otsuka, Roche, Takeda, and Teijin Pharma; research scholarships from AbbVie, Boehringer Ingelheim, Chugai, Daiichi Sankyo, EA Pharma, Eisai, JB, Mitsubishi Tanabe Pharma, Otsuka, Shionogi, Sumitomo Pharma, Teijin Pharma, and Tsumura; and grants from the Ministry of Education, Science and Technology of Japan, the MHLW, and Biogen. Noriko Isobe reports speaker honorarium from Alexion Pharma GK, Biogen Japan, Chugai, CSL Behring, Daiichi Sankyo, Eisai, Mitsubishi Tanabe Pharma, Novartis, Takeda Pharma, Teijin Pharma; research grants from Teijin Pharma, Chugai; and collaborative research with Biogen Japan. Diego Centonze is an employee of the University of Rome Tor Vergata. Sami Fam is an employee and stockholder of Alexion Pharmaceuticals. Adrian Kielhorn is an employee and stockholder of Alexion Pharmaceuticals. Jeffrey Yu is an employee and stockholder of Alexion Pharmaceuticals. Jeroen Jansen is an employee of PRECISIONheor, a consultancy firm, which received funding from Alexion Pharmaceuticals to support this work. Ina Zhang is an employee of PRECISIONheor, a consultancy firm, which received funding from Alexion Pharmaceuticals to support this work.
Figures

References
-
- Hyun JW, Kim Y, Kim SY, Lee MY, Kim SH, Kim HJ. Investigating the presence of interattack astrocyte damage in neuromyelitis optica spectrum disorder: longitudinal analysis of serum glial fibrillary acidic protein. Neurol Neuroimmunol Neuroinflamm. 2021;8(3):e965. doi: 10.1212/NXI.0000000000000965. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

