Inhibition of TBL1 cleavage alleviates doxorubicin-induced cardiomyocytes death by regulating the Wnt/β-catenin signal pathway
- PMID: 38722811
- PMCID: PMC11288742
- DOI: 10.1093/cvr/cvae098
Inhibition of TBL1 cleavage alleviates doxorubicin-induced cardiomyocytes death by regulating the Wnt/β-catenin signal pathway
Abstract
Aims: Doxorubicin (DOX) is a widely used anthracycline anticancer agent; however, its irreversible effects on the heart can result in DOX-induced cardiotoxicity (DICT) after cancer treatment. Unfortunately, the pathophysiology of DICT has not yet been fully elucidated, and there are no effective strategies for its prevention or treatment. In this investigation, the novel role of transducin beta-like protein 1 (TBL1) in developing and regulating DICT was explored.
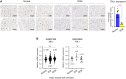
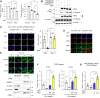
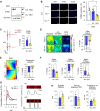
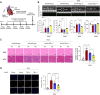
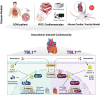
Methods and results: We observed a reduction in TBL1 protein expression levels as well as cleavage events in the transplanted cardiac tissues of patients diagnosed with Dilated Cardiomyopathy and DICT. It was revealed that DOX selectively induces TBL1 cleavage at caspase-3 preferred sites-D125, D136, and D215. Interestingly, overexpression of the uncleaved TBL1 mutant (TBL1uclv) variant reduced apoptosis, effectively preventing DOX-induced cell death. We confirmed that cleaved TBL1 cannot form a complex with β-catenin. As a result, Wnt reporter activity and Wnt target gene expression collectively indicate a decrease in Wnt/β-catenin signalling, leading to DICT progression. Furthermore, the cleaved TBL1 triggered DOX-induced abnormal electrophysiological features and disrupted calcium homeostasis. However, these effects were improved in TBL1uclv-overexpressing human-induced pluripotent stem cell-derived cardiomyocytes. Finally, in a DICT mouse model, TBL1uclv overexpression inhibited the DICT-induced reduction of cardiac contractility and collagen accumulation, ultimately protecting cardiomyocytes from cell death.
Conclusion: Our findings reveal that the inhibition of TBL1 cleavage not only mitigates apoptosis but also enhances cardiomyocyte function, even in the context of DOX administration. Consequently, this study's results suggest that inhibiting TBL1 cleavage may be a novel strategy to ameliorate DICT.
Keywords: Cardiotoxicity; Doxorubicin; Human induced pluripotent stem cell derived cardiomyocytes; Transducin beta-like protein 1; Wnt/β-catenin signal pathway.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interests: none declared.
Figures



References
-
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of Adriamycin cardiotoxicity. Cancer 1973;32:302–314. - PubMed
-
- Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM; Group ESCSD . 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–2801. - PubMed
-
- Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 2018;104:971–977. - PubMed
-
- Haupt LP, Rebs S, Maurer W, Hubscher D, Tiburcy M, Pabel S, Maus A, Kohne S, Tappu R, Haas J, Li Y, Sasse A, Santos CCX, Dressel R, Wojnowski L, Bunt G, Mobius W, Shah AM, Meder B, Wollnik B, Sossalla S, Hasenfuss G, Streckfuss-Bomeke K. Doxorubicin induces cardiotoxicity in a pluripotent stem cell model of aggressive B cell lymphoma cancer patients. Basic Res Cardiol 2022;117:13. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

