Adenosine kinase inhibition protects mice from abdominal aortic aneurysm via epigenetic modulation of VSMC inflammation
- PMID: 38722818
- PMCID: PMC11368124
- DOI: 10.1093/cvr/cvae093
Adenosine kinase inhibition protects mice from abdominal aortic aneurysm via epigenetic modulation of VSMC inflammation
Abstract
Aims: Abdominal aortic aneurysm (AAA) is a common, serious vascular disease with no effective pharmacological treatment. The nucleoside adenosine plays an important role in modulating vascular homeostasis, which prompted us to determine whether adenosine kinase (ADK), an adenosine metabolizing enzyme, modulates AAA formation via control of the intracellular adenosine level, and to investigate the underlying mechanisms.
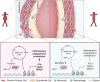
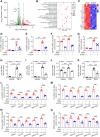
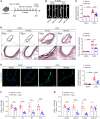
Methods and results: We used a combination of genetic and pharmacological approaches in murine models of AAA induced by calcium chloride (CaCl2) application or angiotensin II (Ang II) infusion to study the role of ADK in the development of AAA. In vitro functional assays were performed by knocking down ADK with adenovirus-short hairpin RNA in human vascular smooth muscle cells (VSMCs), and the molecular mechanisms underlying ADK function were investigated using RNA-sequencing, isotope tracing, and chromatin immunoprecipitation quantitative polymerase chain reaction (ChIP-qPCR). The heterozygous deficiency of ADK protected mice from CaCl2- and Ang II-induced AAA formation. Moreover, specific knockout of ADK in VSMCs prevented Ang II-induced AAA formation, as evidenced by reduced aortic extracellular elastin fragmentation, neovascularization, and aortic inflammation. Mechanistically, ADK knockdown in VSMCs markedly suppressed the expression of inflammatory genes associated with AAA formation, and these effects were independent of adenosine receptors. The metabolic flux and ChIP-qPCR results showed that ADK knockdown in VSMCs decreased S-adenosylmethionine (SAM)-dependent transmethylation, thereby reducing H3K4me3 binding to the promoter regions of the genes that are associated with inflammation, angiogenesis, and extracellular elastin fragmentation. Furthermore, the ADK inhibitor ABT702 protected mice from CaCl2-induced aortic inflammation, extracellular elastin fragmentation, and AAA formation.
Conclusion: Our findings reveal a novel role for ADK inhibition in attenuating AAA via epigenetic modulation of key inflammatory genes linked to AAA pathogenesis.
Keywords: Abdominal aortic aneurysm; Adenosine kinase; Histone methylation; Inflammation; Smooth muscle cells.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: none declared.
Figures





References
-
- Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol 2011;8:92–102. - PubMed
-
- Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol 2019;16:225–242. - PubMed
-
- Sakalihasan N, Michel JB, Katsargyris A, Kuivaniemi H, Defraigne JO, Nchimi A, Powell JT, Yoshimura K, Hultgren R. Abdominal aortic aneurysms. Nat Rev Dis Primers 2018;4:34. - PubMed
-
- Newby D, Forsythe R, McBride O, Robson J, Vesey A, Chalmers R, Burns P, Garden OJ, Semple S, Dweck M, Gray C, MacGillivray T, Wang C, Koutraki YG, Mitchard N, Cooper A, van Beek E, McKillop G, Ho W, Fraser L, Cuthbert H, Hoskins P, Doyle B, Conlisk N, Stuart W, Berry C, Roditi G, Murdoch L, Holdsworth R, Scott E, Milne L, Strachan F, Wee F, Oatey K, Graham C, Murray G, Milne G, Bucukoglu M, Goodman K, Kaczynski J, Shah A, Tambyraja A, Brittenden J, Houston G, Lambie R, Norrie J. Aortic wall inflammation predicts abdominal aortic aneurysm expansion, rupture, and need for surgical repair. Circulation 2017;136:787–797. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

