Integrative species delimitation and five new species of lynx spiders (Araneae, Oxyopidae) in Taiwan
- PMID: 38722906
- PMCID: PMC11081396
- DOI: 10.1371/journal.pone.0301776
Integrative species delimitation and five new species of lynx spiders (Araneae, Oxyopidae) in Taiwan
Erratum in
-
Correction: Integrative species delimitation and five new species of lynx spiders (Araneae, Oxyopidae) in Taiwan.PLoS One. 2024 Dec 19;19(12):e0316369. doi: 10.1371/journal.pone.0316369. eCollection 2024. PLoS One. 2024. PMID: 39700231 Free PMC article.
Abstract
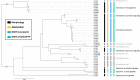
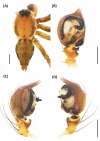
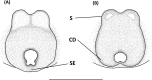
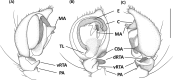
An accurate assessment of species diversity is a cornerstone of biology and conservation. The lynx spiders (Araneae: Oxyopidae) represent one of the most diverse and widespread cursorial spider groups, however their species richness in Asia is highly underestimated. In this study, we revised species diversity with extensive taxon sampling in Taiwan and explored species boundaries based on morphological traits and genetic data using a two-step approach of molecular species delimitation. Firstly, we employed a single COI dataset and applied two genetic distance-based methods: ABGD and ASAP, and two topology-based methods: GMYC and bPTP. Secondly, we further analyzed the lineages that were not consistently delimited, and incorporated H3 to the dataset for a coalescent-based analysis using BPP. A total of eight morphological species were recognized, including five new species, Hamataliwa cordivulva sp. nov., Hamat. leporauris sp. nov., Tapponia auriola sp. nov., T. parva sp. nov. and T. rarobulbus sp. nov., and three newly recorded species, Hamadruas hieroglyphica (Thorell, 1887), Hamat. foveata Tang & Li, 2012 and Peucetia latikae Tikader, 1970. All eight morphological species exhibited reciprocally monophyletic lineages. The results of molecular-based delimitation analyses suggested a variety of species hypotheses that did not fully correspond to the eight morphological species. We found that Hamat. cordivulva sp. nov. and Hamat. foveata showed shallow genetic differentiation in the COI, but they were unequivocally distinguishable according to their genitalia. In contrast, T. parva sp. nov. represented a deep divergent lineage, while differences of genitalia were not detected. This study highlights the need to comprehensively employ multiple evidence and methods to delineate species boundaries and the values of diagnostic morphological characters for taxonomic studies in lynx spiders.
Copyright: © 2024 Lo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






















Similar articles
-
Species delimitation and taxonomic revision of Oxyopes (Araneae: Oxyopidae) of Taiwan, with description of two new species.Zootaxa. 2021 Feb 11;4927(1):zootaxa.4927.1.4. doi: 10.11646/zootaxa.4927.1.4. Zootaxa. 2021. PMID: 33756720
-
Two DNA barcodes and morphology for multi-method species delimitation in Bonnetina tarantulas (Araneae: Theraphosidae).Mol Phylogenet Evol. 2016 Aug;101:176-193. doi: 10.1016/j.ympev.2016.05.003. Epub 2016 May 3. Mol Phylogenet Evol. 2016. PMID: 27150350
-
Violins we see, species we don't… Species delimitation of the spider genus Loxosceles Heineken & Lowe (Araneae: Sicariidae) from North America using morphological and molecular evidence.Zootaxa. 2024 Mar 25;5428(4):527-548. doi: 10.11646/zootaxa.5428.4.4. Zootaxa. 2024. PMID: 39645812
-
Diversity of Pholcus Spiders (Araneae: Pholcidae) in China's Lüliang Mountains: An Integrated Morphological and Molecular Approach.Insects. 2023 Apr 6;14(4):364. doi: 10.3390/insects14040364. Insects. 2023. PMID: 37103180 Free PMC article.
-
Three new species of the spider genus Naphrys Edwards (Araneae, Salticidae) under morphology and molecular data with notes in the distribution of Naphrys acerba (Peckham & Peckham) from Mexico.PeerJ. 2025 Jan 31;13:e18775. doi: 10.7717/peerj.18775. eCollection 2025. PeerJ. 2025. PMID: 39902320 Free PMC article.
Cited by
-
Correction: Integrative species delimitation and five new species of lynx spiders (Araneae, Oxyopidae) in Taiwan.PLoS One. 2024 Dec 19;19(12):e0316369. doi: 10.1371/journal.pone.0316369. eCollection 2024. PLoS One. 2024. PMID: 39700231 Free PMC article.
References
-
- World Spider Catalog. World Spider Catalog. Version 24.5. Natural History Museum Bern; 2023 [cited 23 Oct 2023]. Available: http://wsc.nmbe.ch.
-
- Jocqué R, Dippenaar-Schoeman AS. Spider families of the world. Tervuren, Belgium: Royal Museum for Central Africa; 2006.
-
- Brady AR. The lynx spiders of North America, north of Mexico (Araneae: Oxyopidae). Bull Mus comp Zool Harv. 1964;131: 429–518.
-
- Santos A, Brescovit A. A revision of the Neotropical species of the lynx spider genus Peucetia Thorell 1869 (Araneae: Oxyopidae). Insect Systematics & Evolution. 2003;34: 95–116.
-
- Fink LS. Green Lynx Spider Egg Sacs: Sources of Mortality and the Function of Female Guarding (Araneae, Oxyopidae). The Journal of Arachnology. 1987;15: 231–239.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

