2D to 3D Reconstruction of Boron-Linked Covalent-Organic Frameworks
- PMID: 38723144
- PMCID: PMC11117181
- DOI: 10.1021/jacs.4c02673
2D to 3D Reconstruction of Boron-Linked Covalent-Organic Frameworks
Abstract
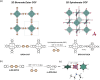
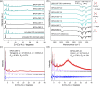
The transformation of two-dimensional (2D) covalent-organic frameworks (COFs) into three-dimensions (3D) is synthetically challenging, and it is typically addressed through interlayer cross-linking of alkene or alkyne bonds. Here, we report the first example of the chemical reconstruction of a 2D COF to a 3D COF with a complete lattice rearrangement facilitated by base-triggered boron hybridization. This chemical reconstruction involves the conversion of trigonal boronate ester linkages to tetrahedral anionic spiroborate linkages. This transformation reticulates the coplanar, closely stacked square cobalt(II) phthalocyanine (PcCo) units into a 3D perpendicular arrangement. As a result, the pore size of COFs expands from 2.45 nm for the initial 2D square lattice (sql) to 3.02 nm in the 3D noninterpenetrated network (nbo). Mechanistic studies reveal a base-catalyzed boronate ester protodeboronation pathway for the formation of the spiroborate structure.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Gui B.; Ding H.; Cheng Y.; Mal A.; Wang C. Structural design and determination of 3D covalent organic frameworks. Trends Chem. 2022, 4 (5), 437–450. 10.1016/j.trechm.2022.01.002. - DOI
-
- Tan K. T.; Ghosh S.; Wang Z.; Wen F.; Rodriguez-San-Miguel D.; Feng J.; Huang N.; Wang W.; Zamora F.; Feng X.; Thomas A.; Jiang D. Covalent organic frameworks. Nat. Rev. Methods Primers 2023, 3, 1.10.1038/s43586-022-00181-z. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

