Mapping interictal discharges using intracranial EEG-fMRI to predict postsurgical outcomes
- PMID: 38723175
- PMCID: PMC11729745
- DOI: 10.1093/brain/awae148
Mapping interictal discharges using intracranial EEG-fMRI to predict postsurgical outcomes
Abstract
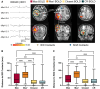
Various subjective and objective methods have been proposed to identify which interictal epileptiform discharge (IED)-related EEG-functional MRI (fMRI) results are more likely to delineate seizure-generating tissue in patients with drug-resistant focal epilepsy for the purposes of surgical planning. In this intracranial EEG-fMRI study, we evaluated the utility of these methods to localize clinically relevant regions preoperatively and compared the extent of resection of these areas to postoperative outcome. Seventy patients admitted for intracranial video-EEG monitoring were recruited for a simultaneous intracranial EEG-fMRI study. For all analyses of blood oxygen level-dependent responses associated with IEDs, an experienced epileptologist identified the most clinically relevant brain activation cluster using available clinical information. The maximum cluster (the cluster with the highest z-score) was also identified for all analyses and assigned to one of three confidence levels (low, medium or high) based on the difference of the peak z-scores between the maximum and second maximum cluster (the cluster with the second highest peak z-value). The distance was measured and compared between the peak voxel of the aforementioned clusters and the electrode contacts where the interictal discharge and seizure onset were recorded. In patients who subsequently underwent epilepsy surgery, the spatial concordance between the aforementioned clusters and the area of resection was determined and compared to postoperative outcome. We evaluated 106 different IEDs in 70 patients. Both subjective (identification of the clinically relevant cluster) and objective (maximum cluster much more significant than the second maximum cluster) methods of culling non-localizing EEG-fMRI activation maps increased the spatial concordance between these clusters and the corresponding IED or seizure onset zone contacts. However, only the objective methods of identifying medium and high confidence maps resulted in a significant association between resection of the peak voxel of the maximum cluster and postoperative outcome. Resection of this area was associated with good postoperative outcomes but was not sufficient for seizure freedom. On the other hand, we found that failure to resect the medium and high confidence maximum clusters was associated with a poor post-surgical outcome (negative predictive value = 1.0, sensitivity = 1.0). Methods to identify higher confidence EEG-fMRI results are needed to localize areas necessary for good postoperative outcomes. However, resection of the peak voxel within higher confidence maximum clusters is not sufficient for good outcomes. Conversely, failure to resect the peak voxel in these clusters is associated with a poor post-surgical outcome.
Keywords: electroencephalography; epilepsy; imaging; markers; seizures; surgery.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures



References
-
- Zijlmans M, Huiskamp G, Hersevoort M, et al. . EEG-fMRI in the preoperative work-up for epilepsy surgery. Brain. 2007;130:2343–2353. - PubMed
-
- Kowalczyk MA, Omidvarnia A, Abbott DF, et al. . Clinical benefit of presurgical EEG-fMRI in difficult-to-localize focal epilepsy: A single-institution retrospective review. Epilepsia. 2020;61:49–60. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

