BEYOND: a randomized controlled trial comparing efficacy and safety of individualized follitropin delta dosing in a GnRH agonist versus antagonist protocol during the first ovarian stimulation cycle
- PMID: 38723189
- PMCID: PMC11759129
- DOI: 10.1093/humrep/deae092
BEYOND: a randomized controlled trial comparing efficacy and safety of individualized follitropin delta dosing in a GnRH agonist versus antagonist protocol during the first ovarian stimulation cycle
Abstract
Study question: How does a gonadotrophin-releasing hormone (GnRH) agonist versus a GnRH antagonist protocol affect ovarian response when using an individualized fixed daily dose of follitropin delta for ovarian stimulation?
Summary answer: The BEYOND trial data demonstrate thatindividualized fixed-dose follitropin delta is effective when used in a GnRH agonist protocol, compared with a GnRH antagonist protocol, in women with anti-Müllerian hormone (AMH) ≤35 pmol/l and no increased risk of ovarian hyperstimulation syndrome (OHSS).
What is known already: The efficacy and safety of an individualized fixed daily dose of follitropin delta (based on body weight and AMH) have been established in randomized controlled trials (RCTs) using a GnRH antagonist protocol. Preliminary study data indicate that individualized follitropin delta is also efficacious in a GnRH agonist protocol (RAINBOW trial, NCT03564509). There are no prospective comparative data using individualized follitropin delta for ovarian stimulation in a GnRH agonist versus a GnRH antagonist protocol.
Study design, size, duration: This is the first randomized, controlled, open-label, multi-centre trial exploring efficacy and safety of individualized follitropin delta dosing in a GnRH agonist versus a GnRH antagonist protocol in participants undergoing their first ovarian stimulation cycle for IVF/ICSI. A total of 437 participants were randomized centrally and stratified by centre and age. The primary endpoint was the number of oocytes retrieved. Secondary endpoints included ongoing pregnancy rates, adverse drug reactions (including OHSS), live births, and neonatal outcomes.
Participants/materials, setting, methods: Participants (18-40 years; AMH ≤35 pmol/l) were enrolled at specialist reproductive health clinics in Austria, Denmark, Israel, Italy, the Netherlands, Norway, and Switzerland. The mean number of oocytes retrieved was compared between the GnRH agonist and antagonist protocols using a negative binomial regression model with age and AMH at screening as factors. Analyses were based on all randomized subjects, using a multiple imputation method for randomized subjects withdrawing before the start of stimulation.
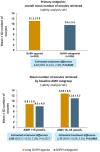
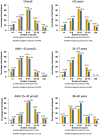
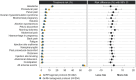
Main results and the role of chance: Of the 437 randomized subjects, 221 were randomized to the GnRH agonist, and 216 were randomized to the GnRH antagonist protocol. The participants had a mean age of 32.3 ± 4.3 years and a mean serum AMH of 16.6 ± 7.8 pmol/l. A total of 202 and 204 participants started ovarian stimulation with follitropin delta in the GnRH agonist and antagonist groups, respectively. The mean number of oocytes retrieved was statistically significantly higher in the agonist group (11.1 ± 5.9) versus the antagonist group (9.6 ± 5.5), with an estimated mean difference of 1.31 oocytes (95% CI: 0.22; 2.40, P = 0.0185). The difference in number of oocytes retrieved was influenced by the patients' age and ovarian reserve, with a greater difference observed in patients aged <35 years and in patients with high ovarian reserve (AMH >15 pmol/l). Both the GnRH agonist and antagonist groups had a similar proportion of cycle cancellations (2.0% [4/202] versus 3.4% [7/204]) and fresh blastocyst transfer cancellations (13.4% [27/202] versus 14.7% [30/204]). The estimated ongoing pregnancy rate per started cycle was numerically higher in the GnRH agonist group (36.9% versus 29.1%; difference: 7.74% [95% CI: -1.49; 16.97, P = 0.1002]). The most commonly reported adverse events (≥1% in either group; headache, OHSS, nausea, pelvic pain, or discomfort and abdominal pain) were similar in both groups. The incidence of early moderate/severe OHSS was low (1.5% for the agonist group versus 2.5% for antagonist groups). Estimated live birth rates per started cycle were 35.8% and 28.7% in the GnRH agonist and antagonist groups, respectively (treatment difference 7.15%; 95% CI: -2.02; 16.31; P = 0.1265). The two treatment groups were comparable with respect to neonatal health data for singletons and twins and for incidence of congenital malformations (2.7% and 3.3% for the GnRH agonist versus antagonist groups, respectively).
Limitations, reasons for caution: All participants had AMH ≤35 pmol/l and were ≤40 years old. Clinicians should remain cautious when using a GnRH agonist protocol in patients with AMH >35 pmol/l (i.e. those with an increased OHSS risk). The incidence of OHSS in the GnRH antagonist group may have been lower if a GnRH agonist trigger had been allowed. Outcomes of transfers with cryopreserved blastocysts were not followed up, therefore the cumulative live birth rates and neonatal outcomes after cryotransfer are unknown.
Wider implications of the findings: In women with AMH ≤35 pmol/l, an individualized fixed daily dose of follitropin delta resulted in a significantly higher number of oocytes retrieved when used in a GnRH agonist protocol compared with a GnRH antagonist protocol, with no additional safety signals observed and no additional risk of OHSS. Live birth rates following ovarian stimulation with individualized follitropin delta were not statistically different between the GnRH protocols; however, the trial was not powered to assess this endpoint. There were no safety concerns with respect to neonatal health after ovarian stimulation with follitropin delta in either protocol.
Study funding/competing interest(s): The trial was funded by Ferring Pharmaceuticals. EE, EP, and MS have no competing interests. AP has received research support from Ferring, and Gedeon Richter, and honoraria or consultation fees from Preglem, Novo Nordisk, Ferring, Gedeon Richter, Cryos, Merck A/S. BC has received consulting fees from Ferring and Merck, and his department received fees from Ferring to cover the costs of patient enrolment. MBS has received support to attend meetings and/or travel from Ferring, and was a board member for FertiPROTEKT e.V. until 2023. JS has received honoraria or consultation fees from Ferring and Merck, and support for attending meetings and/or travel from Ferring, Merck, and GoodLife. TS has received support/travel expenses from Ferring for attending a congress meeting, and participated in an advisory board for Merck. YS has received grants/research support from Ferring and support to attend a professional society congress meeting from Merck. RL and PP are employees of Ferring Pharmaceuticals. PP is a BOD member of PharmaBiome and owns stocks of Takeda Pharmaceuticals.
Trial registration number: ClinicalTrials.gov identifier NCT03809429; EudraCT Number 2017-002783-40.
Trial registration date: 7 April 2019.
Date of first patient’s enrolment: 2 May 2019.
Keywords: GnRH agonist protocol; GnRH antagonist protocol; RCT; follitropin delta; live births; ovarian stimulation; pregnancies.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
E.E., E.P., and M.B.S. have no competing interests. A.P. has received research support from Ferring, and Gedeon Richter, and honoraria or consultation fees from Preglem, Novo Nordisk, Ferring, Gedeon Richter, Cryos, Merck A/S. B.C. has received consulting fees from Ferring and Merck, and his department received fees from Ferring to cover the costs of patient enrolment. M.B.S. has received support to attend meetings and/or travel from Ferring, and was a board member for FertiPROTEKT e. V until 2023. J.S. has received honoraria or consultation fees from Ferring and Merck, and support for attending meetings and/or travel from Ferring, Merck, and GoodLife. T.S. has received support/travel expenses from Ferring for attending a congress meeting, and participated in an advisory board for Merck. Y.S. has received grants/research support from Ferring and support to attend a professional society congress meeting from Merck. R.L. and P.P. are employees of Ferring Pharmaceuticals. P.P. is a BOD member of PharmaBiome and owns stocks of Takeda Pharmaceuticals.
Figures


References
-
- Arce JC, Nyboe Andersen A, Fernandez-Sanchez M, Visnova H, Bosch E, Garcia-Velasco JA, Barri P, de Sutter P, Klein BM, Fauser BC.. Ovarian response to recombinant human follicle-stimulating hormone: a randomized, antiMullerian hormone-stratified, dose-response trial in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril 2014;102:1633–1640.e5. - PubMed
-
- Bosch E, , HavelockJON, , MartinFS, , RasmussenBB, , KleinBM, , MannaertsB, , Arce J-C.. Follitropin delta in repeated ovarian stimulation for IVF: a controlled, assessor-blind Phase 3 safety trial. Reprod Biomed Online 2019;38:195–205. - PubMed
-
- Bosch E, Nyboe Andersen A, Barri P, García-Velasco JA, de Sutter P, Fernández-Sánchez M, Visnova H, Klein BM, Mannaerts B, Arce J-C.. Follicular and endocrine dose responses according to anti-Mullerian hormone levels in IVF patients treated with a novel human recombinant FSH (FE 999049). Clin Endocrinol (Oxf) 2015;83:902–912. - PubMed
-
- Cetrotide SmPC. Cetrotide (Cetrorelix Acetate) 0.25 mg Powder and Solvent for Solution for Injection Summary of Product Characteristics. Darmstadt, Germany: Merck, 2009. www.medicines.org.uk/emc/product/1605 (May 2023, date last accessed).
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

