Clinical and Genomic-Based Decision Support System to Define the Optimal Timing of Allogeneic Hematopoietic Stem-Cell Transplantation in Patients With Myelodysplastic Syndromes
- PMID: 38723212
- PMCID: PMC11328926
- DOI: 10.1200/JCO.23.02175
Clinical and Genomic-Based Decision Support System to Define the Optimal Timing of Allogeneic Hematopoietic Stem-Cell Transplantation in Patients With Myelodysplastic Syndromes
Abstract
Purpose: Allogeneic hematopoietic stem-cell transplantation (HSCT) is the only potentially curative treatment for patients with myelodysplastic syndromes (MDS). Several issues must be considered when evaluating the benefits and risks of HSCT for patients with MDS, with the timing of transplantation being a crucial question. Here, we aimed to develop and validate a decision support system to define the optimal timing of HSCT for patients with MDS on the basis of clinical and genomic information as provided by the Molecular International Prognostic Scoring System (IPSS-M).
Patients and methods: We studied a retrospective population of 7,118 patients, stratified into training and validation cohorts. A decision strategy was built to estimate the average survival over an 8-year time horizon (restricted mean survival time [RMST]) for each combination of clinical and genomic covariates and to determine the optimal transplantation policy by comparing different strategies.
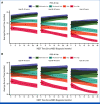
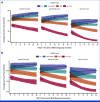
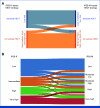
Results: Under an IPSS-M based policy, patients with either low and moderate-low risk benefited from a delayed transplantation policy, whereas in those belonging to moderately high-, high- and very high-risk categories, immediate transplantation was associated with a prolonged life expectancy (RMST). Modeling decision analysis on IPSS-M versus conventional Revised IPSS (IPSS-R) changed the transplantation policy in a significant proportion of patients (15% of patient candidate to be immediately transplanted under an IPSS-R-based policy would benefit from a delayed strategy by IPSS-M, whereas 19% of candidates to delayed transplantation by IPSS-R would benefit from immediate HSCT by IPSS-M), resulting in a significant gain-in-life expectancy under an IPSS-M-based policy (P = .001).
Conclusion: These results provide evidence for the clinical relevance of including genomic features into the transplantation decision making process, allowing personalizing the hazards and effectiveness of HSCT in patients with MDS.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


References
-
- Cutler CS, Lee SJ, Greenberg P, et al. : A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: Delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 104:579-585, 2004 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

