MDA5-autoimmunity and interstitial pneumonitis contemporaneous with the COVID-19 pandemic (MIP-C)
- PMID: 38723554
- PMCID: PMC11090026
- DOI: 10.1016/j.ebiom.2024.105136
MDA5-autoimmunity and interstitial pneumonitis contemporaneous with the COVID-19 pandemic (MIP-C)
Abstract
Background: Anti-MDA5 (Melanoma differentiation-associated protein-5) positive dermatomyositis (MDA5+-DM) is characterised by rapidly progressive interstitial lung disease (ILD) and high mortality. MDA5 is an RNA sensor and a key pattern recognition receptor for the SARS-CoV-2 virus.
Methods: This is a retrospective observational study of a surge in MDA5 autoimmunity, as determined using a 15 muscle-specific autoantibodies (MSAs) panel, between Janurary 2018 and December 2022 in Yorkshire, UK. MDA5-positivity was correlated with clinical features and outcome, and regional SARS-CoV-2 positivity and vaccination rates. Gene expression patterns in COVID-19 were compared with autoimmune lung disease and idiopathic pulmonary fibrosis (IPF) to gain clues into the genesis of the observed MDA5+-DM outbreak.
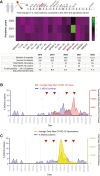
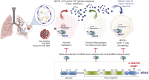
Findings: Sixty new anti-MDA5+, but not other MSAs surged between 2020 and 2022, increasing from 0.4% in 2019 to 2.1% (2020), 4.8% (2021) and 1.7% (2022). Few (8/60) had a prior history of confirmed COVID-19, peak rates overlapped with regional SARS-COV-2 community positivity rates in 2021, and 58% (35/60) had received anti-SARS-CoV-2 vaccines. 25/60 cases developed ILD which rapidly progression with death in 8 cases. Among the 35/60 non-ILD cases, 14 had myositis, 17 Raynaud phenomena and 10 had dermatomyositis spectrum rashes. Transcriptomic studies showed strong IFIH1 (gene encoding for MDA5) induction in COVID-19 and autoimmune-ILD, but not IPF, and IFIH1 strongly correlated with an IL-15-centric type-1 interferon response and an activated CD8+ T cell signature that is an immunologic hallmark of progressive ILD in the setting of systemic autoimmune rheumatic diseases. The IFIH1 rs1990760TT variant blunted such response.
Interpretation: A distinct pattern of MDA5-autoimmunity cases surged contemporaneously with circulation of the SARS-COV-2 virus during COVID-19. Bioinformatic insights suggest a shared immunopathology with known autoimmune lung disease mechanisms.
Funding: This work was supported in part by the National Institute for Health Research (NIHR) Leeds Biomedical Research Centre (BRC), and in part by the National Institutes of Health (NIH) grant R01-AI155696 and pilot awards from the UC Office of the President (UCOP)-RGPO (R00RG2628, R00RG2642 and R01RG3780) to P.G. S.S was supported in part by R01-AI141630 (to P.G) and in part through funds from the American Association of Immunologists (AAI) Intersect Fellowship Program for Computational Scientists and Immunologists.
Keywords: Autoimmune Raynauds; Autoimmune rashes; Coronavirus-19 (Covid-19); Interstitial lung disease (ILD); MDA5-autoimmunity and interstitial pneumonitis contemporaneous with the COVID-19 (MIP-C); Melanoma differentiation-associated protein-5 (MDA5).
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of interests The authors declare that they have no financial conflict of interests for this study.
Figures



Update of
-
MDA5-autoimmunity and Interstitial Pneumonitis Contemporaneous with the COVID-19 Pandemic (MIP-C).medRxiv [Preprint]. 2023 Nov 5:2023.11.03.23297727. doi: 10.1101/2023.11.03.23297727. medRxiv. 2023. Update in: EBioMedicine. 2024 Jun;104:105136. doi: 10.1016/j.ebiom.2024.105136. PMID: 37961408 Free PMC article. Updated. Preprint.
References
-
- Astorri D., Nalotto L., Vaccaro E., et al. AB0715 anti-MDA5 dermatomyositis after BNT162b2 vaccination. Ann Rheum Dis. 2022;81:1484. doi: 10.1136/annrheumdis-2022-eular.3988. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

