A structure-function analysis shows SARS-CoV-2 BA.2.86 balances antibody escape and ACE2 affinity
- PMID: 38723626
- PMCID: PMC11148769
- DOI: 10.1016/j.xcrm.2024.101553
A structure-function analysis shows SARS-CoV-2 BA.2.86 balances antibody escape and ACE2 affinity
Abstract
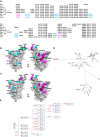
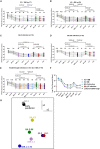
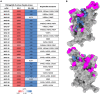
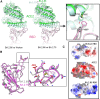
BA.2.86, a recently described sublineage of SARS-CoV-2 Omicron, contains many mutations in the spike gene. It appears to have originated from BA.2 and is distinct from the XBB variants responsible for many infections in 2023. The global spread and plethora of mutations in BA.2.86 has caused concern that it may possess greater immune-evasive potential, leading to a new wave of infection. Here, we examine the ability of BA.2.86 to evade the antibody response to infection using a panel of vaccinated or naturally infected sera and find that it shows marginally less immune evasion than XBB.1.5. We locate BA.2.86 in the antigenic landscape of recent variants and look at its ability to escape panels of potent monoclonal antibodies generated against contemporary SARS-CoV-2 infections. We demonstrate, and provide a structural explanation for, increased affinity of BA.2.86 to ACE2, which may increase transmissibility.
Keywords: ACE2 binding; BA.2.65; SARS-CoV-2; antigenic escape; coronavirus; receptor binding; virus evolution; virus structure.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.R.S. sits on the GSK Vaccines Scientific Advisory Board, consults for AstraZeneca, and is a founder member of RQ Biotechnology. D.I.S. consults for AstraZeneca. Oxford University holds intellectual property related to SARS-CoV-2 mAbs discovered in G.R.S.’s laboratory. S.J.D. is a scientific advisor to the Scottish Parliament on COVID-19.
Figures



References
-
- Obermeyer F., Jankowiak M., Barkas N., Schaffner S.F., Pyle J.D., Yurkovetskiy L., Bosso M., Park D.J., Babadi M., MacInnis B.L., et al. Analysis of 6.4 million SARS-CoV-2 genomes identifies mutations associated with fitness. Science. 2022;376:1327–1332. doi: 10.1126/science.abm1208. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

