Deep mutational scanning of hepatitis B virus reveals a mechanism for cis-preferential reverse transcription
- PMID: 38723628
- PMCID: PMC11127778
- DOI: 10.1016/j.cell.2024.04.008
Deep mutational scanning of hepatitis B virus reveals a mechanism for cis-preferential reverse transcription
Abstract
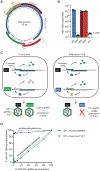
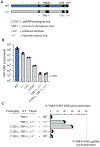
Hepatitis B virus (HBV) is a small double-stranded DNA virus that chronically infects 296 million people. Over half of its compact genome encodes proteins in two overlapping reading frames, and during evolution, multiple selective pressures can act on shared nucleotides. This study combines an RNA-based HBV cell culture system with deep mutational scanning (DMS) to uncouple cis- and trans-acting sequence requirements in the HBV genome. The results support a leaky ribosome scanning model for polymerase translation, provide a fitness map of the HBV polymerase at single-nucleotide resolution, and identify conserved prolines adjacent to the HBV polymerase termination codon that stall ribosomes. Further experiments indicated that stalled ribosomes tether the nascent polymerase to its template RNA, ensuring cis-preferential RNA packaging and reverse transcription of the HBV genome.
Keywords: cis preference; deep mutational scanning; diproline; hepatitis B virus; reverse transcription; ribosome stalling.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Y.Y., W.M.S., and C.M.R. filed a patent application, US 62/741,032, with Rockefeller University on September 19, 2019, entitled “RNA-Based Methods to Launch Hepatitis B Virus Infection.” Patent pending. C.M.R. is a shareholder and member of the scientific advisory board at VIR Biotechnology.
Figures




References
-
- WHO (2021). Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

