Substrate Analogues Entering the Lipoic Acid Salvage Pathway via Lipoate-Protein Ligase 2 Interfere with Staphylococcus aureus Virulence
- PMID: 38724014
- PMCID: PMC11184557
- DOI: 10.1021/acsinfecdis.4c00148
Substrate Analogues Entering the Lipoic Acid Salvage Pathway via Lipoate-Protein Ligase 2 Interfere with Staphylococcus aureus Virulence
Abstract
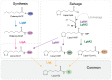
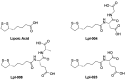
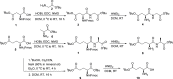
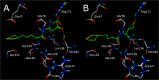
Lipoic acid (LA) is an essential cofactor in prokaryotic and eukaryotic organisms, required for the function of several multienzyme complexes such as oxoacid dehydrogenases. Prokaryotes either synthesize LA or salvage it from the environment. The salvage pathway in Staphylococcus aureus includes two lipoate-protein ligases, LplA1 and LplA2, as well as the amidotransferase LipL. In this study, we intended to hijack the salvage pathway by LA analogues that are transferred via LplA2 and LipL to the E2 subunits of various dehydrogenases, thereby resulting in nonfunctional enzymes that eventually impair viability of the bacterium. Initially, a virtual screening campaign was carried out to identify potential LA analogues that bind to LplA2. Three selected compounds affected S. aureus USA300 growth in minimal medium at concentrations ranging from 2.5 to 10 μg/mL. Further analysis of the most potent compound (Lpl-004) revealed its transfer to E2 subunits of dehydrogenase complexes and a negative impact on its functionality. Growth impairment caused by Lpl-004 treatment was restored by adding products of the lipoate-dependent enzyme complexes. In addition, Caenorhabditis elegans infected with LpL-004-treated USA300 demonstrated a significantly expanded lifespan compared to worms infected with untreated bacteria. Our results provide evidence that LA analogues exploiting the LA salvage pathway represent an innovative strategy for the development of novel antimicrobial substances.
Keywords: LplA2; Staphylococcus aureus; infection model; lipoic acid; salvage pathway; virtual screening.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


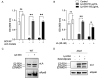
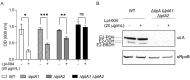
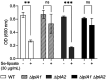
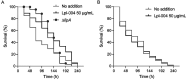
References
-
- Coulter S. N.; Schwan W. R.; Ng E. Y.; Langhorne M. H.; Ritchie H. D.; Westbrock-Wadman S.; Hufnagle W. O.; Folger K. R.; Bayer A. S.; Stover C. K. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol. Microbiol. 1998, 30 (2), 393–404. 10.1046/j.1365-2958.1998.01075.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

