Novel IL-4/HB-EGF-dependent crosstalk between eosinophils and macrophages controls liver regeneration after ischaemia and reperfusion injury
- PMID: 38724220
- PMCID: PMC11347249
- DOI: 10.1136/gutjnl-2024-332033
Novel IL-4/HB-EGF-dependent crosstalk between eosinophils and macrophages controls liver regeneration after ischaemia and reperfusion injury
Abstract
Objective: Previous studies indicate that eosinophils are recruited into the allograft following orthotopic liver transplantation and protect from ischaemia reperfusion (IR) injury. In the current studies, we aim to explore whether their protective function could outlast during liver repair.
Design: Eosinophil-deficient mice and adoptive transfer of bone marrow-derived eosinophils (bmEos) were employed to investigate the effects of eosinophils on tissue repair and regeneration after hepatic IR injury. Aside from exogenous cytokine or neutralising antibody treatments, mechanistic studies made use of a panel of mouse models of eosinophil-specific IL-4/IL-13-deletion, cell-specific IL-4rα-deletion in liver macrophages and hepatocytes and macrophage-specific deletion of heparin-binding epidermal growth factor-like growth factor (hb-egf).
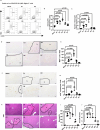
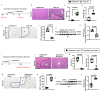
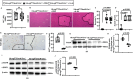
Result: We observed that eosinophils persisted over a week following hepatic IR injury. Their peak accumulation coincided with that of hepatocyte proliferation. Functional studies showed that eosinophil deficiency was associated with a dramatic delay in liver repair, which was normalised by the adoptive transfer of bmEos. Mechanistic studies demonstrated that eosinophil-derived IL-4, but not IL-13, was critically involved in the reparative function of these cells. The data further revealed a selective role of macrophage-dependent IL-4 signalling in liver regeneration. Eosinophil-derived IL-4 stimulated macrophages to produce HB-EGF. Moreover, macrophage-specific hb-egf deletion impaired hepatocyte regeneration after IR injury.
Conclusion: Together, these studies uncovered an indispensable role of eosinophils in liver repair after acute injury and identified a novel crosstalk between eosinophils and macrophages through the IL-4/HB-EGF axis.
Keywords: growth factors; immunology in hepatology; ischaemia-reperfusion; liver regeneration; macrophages.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
