Neurophysiological features of STN LFP underlying sleep fragmentation in Parkinson's disease
- PMID: 38724231
- PMCID: PMC7616489
- DOI: 10.1136/jnnp-2023-331979
Neurophysiological features of STN LFP underlying sleep fragmentation in Parkinson's disease
Abstract
Background: Sleep fragmentation is a persistent problem throughout the course of Parkinson's disease (PD). However, the related neurophysiological patterns and the underlying mechanisms remained unclear.
Method: We recorded subthalamic nucleus (STN) local field potentials (LFPs) using deep brain stimulation (DBS) with real-time wireless recording capacity from 13 patients with PD undergoing a one-night polysomnography recording, 1 month after DBS surgery before initial programming and when the patients were off-medication. The STN LFP features that characterised different sleep stages, correlated with arousal and sleep fragmentation index, and preceded stage transitions during N2 and REM sleep were analysed.
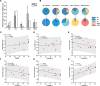
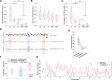
Results: Both beta and low gamma oscillations in non-rapid eye movement (NREM) sleep increased with the severity of sleep disturbance (arousal index (ArI)-betaNREM: r=0.9, p=0.0001, sleep fragmentation index (SFI)-betaNREM: r=0.6, p=0.0301; SFI-gammaNREM: r=0.6, p=0.0324). We next examined the low-to-high power ratio (LHPR), which was the power ratio of theta oscillations to beta and low gamma oscillations, and found it to be an indicator of sleep fragmentation (ArI-LHPRNREM: r=-0.8, p=0.0053; ArI-LHPRREM: r=-0.6, p=0.0373; SFI-LHPRNREM: r=-0.7, p=0.0204; SFI-LHPRREM: r=-0.6, p=0.0428). In addition, long beta bursts (>0.25 s) during NREM stage 2 were found preceding the completion of transition to stages with more cortical activities (towards Wake/N1/REM compared with towards N3 (p<0.01)) and negatively correlated with STN spindles, which were detected in STN LFPs with peak frequency distinguishable from long beta bursts (STN spindle: 11.5 Hz, STN long beta bursts: 23.8 Hz), in occupation during NREM sleep (β=-0.24, p<0.001).
Conclusion: Features of STN LFPs help explain neurophysiological mechanisms underlying sleep fragmentations in PD, which can inform new intervention for sleep dysfunction.
Trial registration number: NCT02937727.
Keywords: PARKINSON'S DISEASE; SLEEP.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: Luming Li and Hongwei Hao serve on the scientific advisory board for Beijing Pins Medical Co., Ltd and are listed as inventors in issued patents and patent applications on the deep brain stimulator used in this work.
Figures





References
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
