Human iPSC-Derived Neurons with Reliable Synapses and Large Presynaptic Action Potentials
- PMID: 38724283
- PMCID: PMC11170674
- DOI: 10.1523/JNEUROSCI.0971-23.2024
Human iPSC-Derived Neurons with Reliable Synapses and Large Presynaptic Action Potentials
Abstract
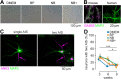
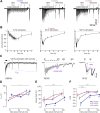
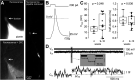
Understanding the function of the human brain requires determining basic properties of synaptic transmission in human neurons. One of the most fundamental parameters controlling neurotransmitter release is the presynaptic action potential, but its amplitude and duration remain controversial. Presynaptic action potentials have so far been measured with high temporal resolution only in a limited number of vertebrate but not in human neurons. To uncover properties of human presynaptic action potentials, we exploited recently developed tools to generate human glutamatergic neurons by transient expression of Neurogenin 2 (Ngn2) in pluripotent stem cells. During maturation for 3 to 9 weeks of culturing in different established media, the proportion of cells with multiple axon initial segments decreased, while the amount of axonal tau protein and neuronal excitability increased. Super-resolution microscopy revealed the alignment of the pre- and postsynaptic proteins, Bassoon and Homer. Synaptic transmission was surprisingly reliable at frequencies of 20, 50, and 100 Hz. The synchronicity of synaptic transmission during high-frequency transmission increased during 9 weeks of neuronal maturation. To analyze the mechanisms of synchronous high-frequency glutamate release, we developed direct presynaptic patch-clamp recordings from human neurons. The presynaptic action potentials had large overshoots to ∼25 mV and short durations of ∼0.5 ms. Our findings show that Ngn2-induced neurons represent an elegant model system allowing for functional, structural, and molecular analyses of glutamatergic synaptic transmission with high spatiotemporal resolution in human neurons. Furthermore, our data predict that glutamatergic transmission is mediated by large and rapid presynaptic action potentials in the human brain.
Keywords: action potential; human; iPSC; presynaptic.
Copyright © 2024 Bullmann et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
