Bortezomib exerts its anti-cancer activity through the regulation of Skp2/p53 axis in non-melanoma skin cancer cells and C. elegans
- PMID: 38724504
- PMCID: PMC11082213
- DOI: 10.1038/s41420-024-01992-7
Bortezomib exerts its anti-cancer activity through the regulation of Skp2/p53 axis in non-melanoma skin cancer cells and C. elegans
Abstract
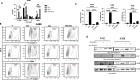
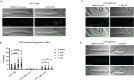
Non-melanoma skin cancer (NMSC), encompassing basal and squamous cell carcinoma, is the most prevalent cancer in the United States. While surgical removal remains the conventional therapy with a 95% 5-year cure rate, there is a growing interest in exploring alternative treatment strategies. In this study, we investigated the role of Bortezomib (BTZ), a proteasome inhibitor, in NMSC. Using two NMSC cell lines (A431 and A388), we examined the effects of BTZ treatment. Our results demonstrated that 48 h of BTZ treatment led to downregulating Skp2 expression in both A431 and A388 cells while upregulating p53 expression, specifically in A388 cells. These alterations resulted in impaired cellular growth and caspase-dependent cell death. Silencing Skp2 in A388 cells with siRNA confirmed the upregulation of p53 as a direct target. Furthermore, BTZ treatment increased the Bax to Bcl-2 ratio, promoting mitochondrial permeability and the subsequent release of cytochrome C, thereby activating caspases. We also found that BTZ exerted its antitumor effects by generating reactive oxygen species (ROS), as blocking ROS production significantly reduced BTZ-induced apoptotic cell death. Interestingly, BTZ treatment induced autophagy, which is evident from the increased expression of microtubule-associated proteins nucleoporin p62 and LC-3A/B. In addition to cell lines, we assessed the impact of BTZ in an in vivo setting using Caenorhabditis elegans (C. elegans). Our findings demonstrated that BTZ induced germline apoptosis in worms even at low concentrations. Notably, this increased apoptosis was mediated through the activity of CEP-1, the worm's counterpart to mammalian p53. In summary, our study elucidated the molecular mechanism underlying BTZ-induced apoptosis in NMSC cell lines and C. elegans. By targeting the skp2/p53 axis, inducing mitochondrial permeability, generating ROS, and promoting autophagy, BTZ demonstrates promising anti-cancer activity in NMSC. These findings provide novel insights into potential therapeutic strategies for controlling the unregulated growth of NMSC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells.J Biol Chem. 2003 Sep 5;278(36):33714-23. doi: 10.1074/jbc.M302559200. Epub 2003 Jun 23. J Biol Chem. 2003. PMID: 12821677
-
Combined bortezomib-based chemotherapy and p53 gene therapy using hollow mesoporous silica nanospheres for p53 mutant non-small cell lung cancer treatment.Biomater Sci. 2016 Dec 20;5(1):77-88. doi: 10.1039/c6bm00449k. Biomater Sci. 2016. PMID: 27822577
-
Targeting MUC1-C is synergistic with bortezomib in downregulating TIGAR and inducing ROS-mediated myeloma cell death.Blood. 2014 May 8;123(19):2997-3006. doi: 10.1182/blood-2013-11-539395. Epub 2014 Mar 14. Blood. 2014. PMID: 24632713 Free PMC article.
-
Endoplasmic-reticulum stress pathway-associated mechanisms of action of proteasome inhibitors in multiple myeloma.Int J Hematol. 2016 Sep;104(3):273-80. doi: 10.1007/s12185-016-2016-0. Epub 2016 May 12. Int J Hematol. 2016. PMID: 27169614 Review.
-
Epigenetic Alterations as Vital Aspects of Bortezomib Molecular Action.Cancers (Basel). 2023 Dec 23;16(1):84. doi: 10.3390/cancers16010084. Cancers (Basel). 2023. PMID: 38201512 Free PMC article. Review.
Cited by
-
Thiostrepton induces apoptotic cell death at the level of BCL-2/CED-9 in C. elegans.Sci Rep. 2025 Jul 8;15(1):24415. doi: 10.1038/s41598-025-09446-5. Sci Rep. 2025. PMID: 40628925 Free PMC article.
References
-
- Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. 2004;4:23–35. - PubMed
-
- Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–80. - PubMed
-
- Xiang F, Lucas R, Hales S, Neale R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978-2012: empirical relationships. JAMA Dermatol. 2014;150:1063–71. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

