MnO2/TiO2-Catalyzed ozonolysis: enhancing Pentachlorophenol degradation and understanding intermediates
- PMID: 38725018
- PMCID: PMC11080107
- DOI: 10.1186/s13065-024-01194-3
MnO2/TiO2-Catalyzed ozonolysis: enhancing Pentachlorophenol degradation and understanding intermediates
Abstract
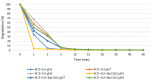
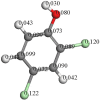
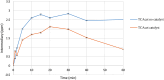
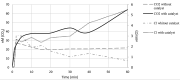
Pentachlorophenol is a pesticide widely known for its harmful effects on sewage, causing harm to the environment. In previous studies, our group identified adsorption as a crucial factor in catalytic ozonation processes, and subsequent observations revealed the catalyst's role in reducing toxicity during degradation. In this research, we quantified organochlorine intermediates and low molecular weight organic acids generated under optimal pH conditions (pH 9), with and without the catalyst. Additionally, we assessed the reactivity of these intermediates through theoretical calculations. Our findings indicate that the catalyst reduces the duration of intermediates. Additionally, the presence of CO2 suggests enhanced mineralization of pentachlorophenol, a process notably facilitated by the catalyst. Theoretical calculations, such as Fukui analysis, offer insights into potential pathways for the dechlorination of aromatic molecules by radicals like OH, indicating the significance of this pathway.
Keywords: Dechloration; Fukui; MnO2/TiO2; Ozonation; Pentachlorophenol.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures











References
-
- Chen K-C, Wang Y-H. The effects of Fe–Mn oxide and TiO2/α-Al2O3 on the formation of disinfection by-products in catalytic ozonation. Chem Eng J. 2014;253:84–92. doi: 10.1016/j.cej.2014.04.111. - DOI
-
- WHO. Pentachlorophenol: health and safety guide. World Health Organization; 1998.
LinkOut - more resources
Full Text Sources
