Reduced cerebrospinal fluid motion in patients with Parkinson's disease revealed by magnetic resonance imaging with low b-value diffusion weighted imaging
- PMID: 38725029
- PMCID: PMC11080257
- DOI: 10.1186/s12987-024-00542-8
Reduced cerebrospinal fluid motion in patients with Parkinson's disease revealed by magnetic resonance imaging with low b-value diffusion weighted imaging
Abstract
Background: Parkinson's disease is characterized by dopamine-responsive symptoms as well as aggregation of α-synuclein protofibrils. New diagnostic methods assess α-synuclein aggregation characteristics from cerebrospinal fluid (CSF) and recent pathophysiologic mechanisms suggest that CSF circulation disruptions may precipitate α-synuclein retention. Here, diffusion-weighted MRI with low-to-intermediate diffusion-weightings was applied to test the hypothesis that CSF motion is reduced in Parkinson's disease relative to healthy participants.
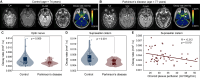
Methods: Multi-shell diffusion weighted MRI (spatial resolution = 1.8 × 1.8 × 4.0 mm) with low-to-intermediate diffusion weightings (b-values = 0, 50, 100, 200, 300, 700, and 1000 s/mm2) was applied over the approximate kinetic range of suprasellar cistern fluid motion at 3 Tesla in Parkinson's disease (n = 27; age = 66 ± 6.7 years) and non-Parkinson's control (n = 32; age = 68 ± 8.9 years) participants. Wilcoxon rank-sum tests were applied to test the primary hypothesis that the noise floor-corrected decay rate of CSF signal as a function of b-value, which reflects increasing fluid motion, is reduced within the suprasellar cistern of persons with versus without Parkinson's disease and inversely relates to choroid plexus activity assessed from perfusion-weighted MRI (significance-criteria: p < 0.05).
Results: Consistent with the primary hypothesis, CSF decay rates were higher in healthy (D = 0.00673 ± 0.00213 mm2/s) relative to Parkinson's disease (D = 0.00517 ± 0.00110 mm2/s) participants. This finding was preserved after controlling for age and sex and was observed in the posterior region of the suprasellar cistern (p < 0.001). An inverse correlation between choroid plexus perfusion and decay rate in the voxels within the suprasellar cistern (Spearman's-r=-0.312; p = 0.019) was observed.
Conclusions: Multi-shell diffusion MRI was applied to identify reduced CSF motion at the level of the suprasellar cistern in adults with versus without Parkinson's disease; the strengths and limitations of this methodology are discussed in the context of the growing literature on CSF flow.
Keywords: Cerebrospinal fluid; Choroid plexus; DWI; Glymphatic; Parkinson’s; Suprasellar cistern; α-synuclein.
© 2024. The Author(s).
Conflict of interest statement
No authors declare any relevant conflicts or disclosures regarding the work presented in this manuscript. Manus J. Donahue receives research related support from the National Institutes of Health (NINDS, NCI, NIA, NCCIH, NINR, and NHLBI), Philips Healthcare and is a paid consultant for Graphite Bio, Pfizer Inc, Global Blood Therapeutics, and LymphaTouch. He is a paid advisory board member for Novartis and bluebird bio and receives research funding from the National Institutes of Health and Pfizer Inc. Manus J. Donahue is also the CEO of Biosight Inc which operates as a clinical research organization and provides healthcare technology vendor services. Ciaran Considine?s financial disclosures include those associated with his private consulting practice, NeuropsyConsulting LLC, i.e., Forensic Consulting with Park Dietz & Associates, clinical-research advisory panel member with MDisrupt, external advisory board for HD Genetics. He also receives academic-research grant support from the NIH, NIA, and DoD, and acts as PI on an investigator-initiated clinical study funded by Acadia. Daniel O. Claassen has received research support from the NIH/NINDS/NIA/NICHD/NCCIH, Department of Defense, Griffin Family Foundation, and Huntington Disease Society of America; he has received pharmaceutical grant support from AbbVie, Alterity, Acadia, Biogen, BMS, Cerecour, Eli Lilly, Genentech-Roche, Lundbeck, Jazz Pharmaceuticals, Neurocrine, Teva Neuroscience, Wave Life Sciences, UniQure, and Vaccinex. He has received personal fees for consulting from Acadia, Alterity, Adamas, Anexon, Ceruvel, Lundbeck, Neurocrine, Spark, Uniqure, and Teva Neuroscience.
Figures





Update of
-
Reduced suprasellar cistern cerebrospinal fluid motion in patients with Parkinson's disease revealed by magnetic resonance imaging with dynamic cycling of diffusion weightings.Res Sq [Preprint]. 2023 Sep 7:rs.3.rs-3311121. doi: 10.21203/rs.3.rs-3311121/v1. Res Sq. 2023. Update in: Fluids Barriers CNS. 2024 May 9;21(1):40. doi: 10.1186/s12987-024-00542-8. PMID: 37720044 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

