A new era of LMCT: leveraging ligand-to-metal charge transfer excited states for photochemical reactions
- PMID: 38725519
- PMCID: PMC11079626
- DOI: 10.1039/d3sc05268k
A new era of LMCT: leveraging ligand-to-metal charge transfer excited states for photochemical reactions
Abstract
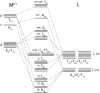
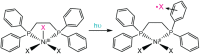
Ligand-to-metal charge transfer (LMCT) excited states are capable of undergoing a wide array of photochemical reactions, yet receive minimal attention compared to other charge transfer excited states. This work provides general criteria for designing transition metal complexes that exhibit low energy LMCT excited states and routes to drive photochemistry from these excited states. General design principles regarding metal identity, oxidation state, geometry, and ligand sets are summarized. Fundamental photoreactions from these states including visible light-induced homolysis, excited state electron transfer, and other photoinduced chemical transformations are discussed and key design principles for enabling these photochemical reactions are further highlighted. Guided by these fundamentals, this review outlines critical considerations for the future design and application of coordination complexes with LMCT excited states.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
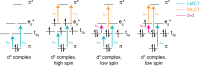


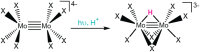


Similar articles
-
Excited State Bond Homolysis of Vanadium(V) Photocatalysts for Alkoxy Radical Generation.J Phys Chem A. 2024 Sep 12;128(36):7609-7619. doi: 10.1021/acs.jpca.4c04250. Epub 2024 Aug 30. J Phys Chem A. 2024. PMID: 39213596
-
Delayed fluorescence from a zirconium(IV) photosensitizer with ligand-to-metal charge-transfer excited states.Nat Chem. 2020 Apr;12(4):345-352. doi: 10.1038/s41557-020-0430-7. Epub 2020 Mar 16. Nat Chem. 2020. PMID: 32203439
-
Energy cascades, excited state dynamics, and photochemistry in cob(III)alamins and ferric porphyrins.Acc Chem Res. 2015 Mar 17;48(3):860-7. doi: 10.1021/ar5004016. Epub 2015 Mar 5. Acc Chem Res. 2015. PMID: 25741574
-
Emission Spectroscopy as a Probe into Photoinduced Intramolecular Electron Transfer in Polyazine Bridged Ru(II),Rh(III) Supramolecular Complexes.Materials (Basel). 2010 Aug 11;3(8):4328-4354. doi: 10.3390/ma3084328. Materials (Basel). 2010. PMID: 28883332 Free PMC article. Review.
-
Photoinduced ligand-to-metal charge transfer (LMCT) in organic synthesis: reaction modes and research advances.Chem Commun (Camb). 2025 Jan 28;61(10):1944-1961. doi: 10.1039/d4cc06099g. Chem Commun (Camb). 2025. PMID: 39760393 Review.
Cited by
-
Unraveling the Roles of Amines in Atom Transfer Radical Polymerization in the Dark.J Am Chem Soc. 2025 Apr 16;147(15):12562-12573. doi: 10.1021/jacs.4c18496. Epub 2025 Apr 2. J Am Chem Soc. 2025. PMID: 40173322 Free PMC article.
-
A Near-Infrared-II Luminescent and Photoactive Vanadium(II) Complex with a 760 ns Excited State Lifetime.J Am Chem Soc. 2025 Jun 18;147(24):20833-20842. doi: 10.1021/jacs.5c04471. Epub 2025 Jun 3. J Am Chem Soc. 2025. PMID: 40462271 Free PMC article.
-
Precisely metal doped nanographenes via a carbaporphyrin approach.Nat Commun. 2025 Feb 11;16(1):1534. doi: 10.1038/s41467-025-56828-4. Nat Commun. 2025. PMID: 39934131 Free PMC article.
-
Formation of polysulfides as a smart strategy to selectively detect H2S in a Bi(iii)-based MOF material.Chem Sci. 2025 Feb 19;16(13):5483-5492. doi: 10.1039/d4sc07144a. eCollection 2025 Mar 26. Chem Sci. 2025. PMID: 40012690 Free PMC article.
-
Quantum Mechanics MP2 and CASSCF Study of Coordinate Quasi-Double Bonds in Cobalt(II) Complexes as Single Molecule Magnets.Nanomaterials (Basel). 2025 Jun 17;15(12):938. doi: 10.3390/nano15120938. Nanomaterials (Basel). 2025. PMID: 40559301 Free PMC article.
References
-
- Chábera P. Lindh L. Rosemann N. W. Prakash O. Uhlig J. Yartsev A. Wärnmark K. Sundström V. Persson P. Photofunctionality of Iron(III) N-Heterocyclic Carbenes and Related d5 Transition Metal Complexes. Coord. Chem. Rev. 2021;426:213517. doi: 10.1016/j.ccr.2020.213517. doi: 10.1016/j.ccr.2020.213517. - DOI - DOI
Publication types
LinkOut - more resources
Full Text Sources

