Targeting hypoxia signaling pathways in angiogenesis
- PMID: 38725568
- PMCID: PMC11079266
- DOI: 10.3389/fphys.2024.1408750
Targeting hypoxia signaling pathways in angiogenesis
Abstract
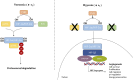
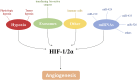
Oxygen (O2) supply is constantly maintained by the vascular network for a proper tissue oxygenation. Hypoxia is the result of an increased O2 demand and/or decreased supply and is common in both physiological conditions and human diseases. Angiogenesis is one of the adaptive responses to hypoxia and is mainly regulated by the hypoxia-inducible factors, HIFs. These heterodimeric transcription factors are composed of one of three O2-dependent α subunits (HIF-1, HIF-2, and HIF-3) and a constitutively expressed O2-insensitive subunit (HIF-1β). Among them HIF-1α is the most characterized and its activity is tightly controlled. Under hypoxia, its intracellular accumulation triggers the transcription of several genes, involved in cell survival/proliferation, autophagy, apoptosis, cell metabolism, and angiogenesis. HIF pathway is also modulated by specific microRNAs (miRNAs), thus resulting in the variation of several cellular responses, including alteration of the angiogenic process. The pro-angiogenic activity of HIF-1α is not restricted to endothelial cells, as it also affects the behavior of other cell types, including tumor and inflammatory/immune cells. In this context, exosomes play a crucial role in cell-cell communication by transferring bio-active cargos such as mRNAs, miRNAs, and proteins (e.g., VEGFA mRNA, miR210, HIF-1α). This minireview will provide a synopsis of the multiple factors able to modulate hypoxia-induced angiogenesis especially in the tumor microenvironment context. Targeting hypoxia signaling pathways by up-to-date approaches may be relevant in the design of therapeutic strategies in those pathologies where angiogenesis is dysregulated.
Keywords: HIFs; angiogenesis; endothelial cells; exosomes; hypoxia; immune cells; miRNAs.
Copyright © 2024 Monaci, Coppola, Filippi, Falsini, Carraro and Naldini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bartoszewski R., Moszyńska A., Serocki M., Cabaj A., Polten A., Ochocka R., et al. (2019). Primary endothelial cell–specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. FASEB J. 33 (7), 7929–7941. 10.1096/fj.201802650RR - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

