In the fed state, autophagy plays a crucial role in assisting the insect vector Rhodnius prolixus mobilize TAG reserves under forced flight activity
- PMID: 38725570
- PMCID: PMC11079428
- DOI: 10.3389/fphys.2024.1352766
In the fed state, autophagy plays a crucial role in assisting the insect vector Rhodnius prolixus mobilize TAG reserves under forced flight activity
Abstract
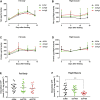
Autophagy is a cellular degradation pathway mediated by highly conserved autophagy-related genes (Atgs). In our previous work, we showed that inhibiting autophagy under starvation conditions leads to significant physiological changes in the insect vector of Chagas disease Rhodnius prolixus; these changes include triacylglycerol (TAG) retention in the fat body, reduced survival and impaired locomotion and flight capabilities. Herein, because it is known that autophagy can be modulated in response to various stimuli, we further investigated the role of autophagy in the fed state, following blood feeding. Interestingly, the primary indicator for the presence of autophagosomes, the lipidated form of Atg8 (Atg8-II), displayed 20%-50% higher autophagic activation in the first 2 weeks after feeding compared to the third week when digestion was complete. Despite the elevated detection of autophagosomes, RNAi-mediated suppression of RpAtg6 and RpAtg8 did not cause substantial changes in TAG or protein levels in the fat body or the flight muscle during blood digestion. We also found that knockdown of RpAtg6 and RpAtg8 led to modest modulations in the gene expression of essential enzymes involved in lipid metabolism and did not significantly stimulate the expression of the chaperones BiP and PDI, which are the main effectors of the unfolded protein response. These findings indicate that impaired autophagy leads to slight disturbances in lipid metabolism and general cell proteostasis. However, the ability of insects to fly during forced flight until exhaustion was reduced by 60% after knockdown of RpAtg6 and RpAtg8. This change was accompanied by TAG and protein increases as well as decreased ATP levels in the fat body and flight muscle, indicating that autophagy during digestion, i.e., under fed conditions, is necessary to sustain high-performance activity.
Keywords: Chagas disease; Rhodnius prolixus; autophagy; flight activity; lipophagy.
Copyright © 2024 Santos-Araujo, Gomes, Carvalho-Kelly, Meyer-Fernandes, Gondim and Ramos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






References
-
- Almeida-Oliveira F., Santos-Araujo S., Carvalho-Kelly L. F., Macedo-Silva A., Meyer-Fernandes J. R., Gondim K. C., et al. (2023). ATP synthase affects lipid metabolism in the kissing bug Rhodnius prolixus beyond its role in energy metabolism. Insect Biochem. Mol. Biol. 158, 103956. 10.1016/j.ibmb.2023.103956 - DOI - PubMed
-
- Alves-Bezerra M., De Paula I. F., Medina J. M., Silva-Oliveira G., Medeiros J. S., Gäde G., et al. (2016a). Adipokinetic hormone receptor gene identification and its role in triacylglycerol metabolism in the blood-sucking insect Rhodnius prolixus . Insect Biochem. Mol. Biol. 69, 51–60. 10.1016/j.ibmb.2015.06.013 - DOI - PubMed
-
- Alves-Bezerra M., Klett E. L., De Paula I. F., Ramos I. B., Coleman R. A., Gondim K. C. (2016b). Long-chain acyl-CoA synthetase 2 knockdown leads to decreased fatty acid oxidation in fat body and reduced reproductive capacity in the insect Rhodnius prolixus . Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1861, 650–662. 10.1016/j.bbalip.2016.04.007 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

