Targeting PD-L1 in solid cancer with myeloid cells expressing a CAR-like immune receptor
- PMID: 38726005
- PMCID: PMC11079125
- DOI: 10.3389/fimmu.2024.1380065
Targeting PD-L1 in solid cancer with myeloid cells expressing a CAR-like immune receptor
Abstract
Introduction: Solid cancers Myeloid cells are prevalent in solid cancers, but they frequently exhibit an anti-inflammatory pro-tumor phenotype that contribute to the immunosuppressive tumor microenvironment (TME), which hinders the effectiveness of cancer immunotherapies. Myeloid cells' natural ability of tumor trafficking makes engineered myeloid cell therapy an intriguing approach to tackle the challenges posed by solid cancers, including tumor infiltration, tumor cell heterogenicity and the immunosuppressive TME. One such engineering approach is to target the checkpoint molecule PD-L1, which is often upregulated by solid cancers to evade immune responses.
Method: Here we devised an adoptive cell therapy strategy based on myeloid cells expressing a Chimeric Antigen Receptor (CAR)-like immune receptor (CARIR). The extracellular domain of CARIR is derived from the natural inhibitory receptor PD-1, while the intracellular domain(s) are derived from CD40 and/or CD3ζ. To assess the efficacy of CARIR-engineered myeloid cells, we conducted proof-of-principle experiments using co-culture and flow cytometry-based phagocytosis assays in vitro. Additionally, we employed a fully immune-competent syngeneic tumor mouse model to evaluate the strategy's effectiveness in vivo.
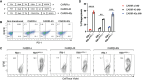
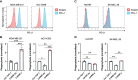
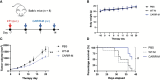
Result: Co-culturing CARIR-expressing human monocytic THP-1 cells with PD-L1 expressing target cells lead to upregulation of the costimulatory molecule CD86 along with expression of proinflammatory cytokines TNF-1α and IL-1β. Moreover, CARIR expression significantly enhanced phagocytosis of multiple PD-L1 expressing cancer cell lines in vitro. Similar outcomes were observed with CARIR-expressing human primary macrophages. In experiments conducted in syngeneic BALB/c mice bearing 4T1 mammary tumors, infusing murine myeloid cells that express a murine version of CARIR significantly slowed tumor growth and prolonged survival.
Conclusion: Taken together, these results demonstrate that adoptive transfer of PD-1 CARIR-engineered myeloid cells represents a promising strategy for treating PD-L1 positive solid cancers.
Keywords: PD-1; PD-L1; adoptive cell therapy; chimeric antigen receptor (CAR); macrophages; phagocytosis; solid cancer.
Copyright © 2024 Myers Chen, Grun, Gautier, Venkatesha, Maddox, Zhang and Andersen.
Conflict of interest statement
Authors KC, DG, BG, SV, MM, A-HZ, and PA are employees and/or shareholders of Vita Therapeutics, Inc. A-HZ, PA, KC, and DG are inventors of pending patents involving the generation and use of CARIR-modified myeloid cells for treating cancer.
Figures


References
-
- U.S. Department of Health and Human Services. Center for Disease Control and Prevention. National Cancer Institute . U.S. Cancer Statistics Data Visualizations Tool, based on 2020 submission data (1999-2018). Available online at: https://gis.cdc.gov/Cancer/USCS/#/AtAGlance/.
-
- Bauml J, Barton D, Ronczka A, Cushing D, Klichinsky M, Dees EC. A phase 1, first in human (FIH) study of adenovirally transduced autologous macrophages engineered to contain an anti-HER2 chimeric antigen receptor (CAR) in subjects with HER2 overexpressing solid tumors. Cytotherapy. (2021) 23. doi: 10.1016/s1465324921004205 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

