Zuranolone for treatment of major depressive disorder: a systematic review and meta-analysis
- PMID: 38726035
- PMCID: PMC11079210
- DOI: 10.3389/fnins.2024.1361692
Zuranolone for treatment of major depressive disorder: a systematic review and meta-analysis
Abstract
Background: Current treatment modalities for Major Depressive Disorder have variable efficacies and a variety of side effects. To amend this, many trials for short term, well tolerated monotherapies are underway. One such option is Zuranolone (SAGE-217), which is a recent FDA approved antidepressant for Post Partum depression (PPD) and is undergoing clinical trials for PPD, major depressive disorder (MDD) and essential tremors (ET).
Objectives: Pool currently available data that compare Zuranolone to Placebo for the treatment of Major Depressive Disorder and evaluate its efficacy and safety profile.
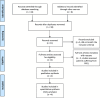
Methods: We retrieved data from PUBMED and SCOPUS from inception to July 2023. We included articles comparing Zuranolone or SAGE 217 with placebo in patients suffering from Major Depressive Disorder. Review Manager 5.4 was used to analyze the outcomes including changes in the Hamilton Depression Rating Scale (HAM-D), Hamilton Anxiety Rating Scale (HAM-A) and Montgomery-Åsberg Depression Rating Scale (MADRS) scores from baseline as well as any treatment emergent adverse events (TEAEs) and severe adverse events.
Results: Our review analyzed 4 trials and the data of 1,357 patients. Patients treated with Zuranolone indicated a statistically significant effect in the change from baseline in HAM-D score (p = 0.0009; MD [95% CI]: -2.03 [-3.23, -0.84]) as well as in MADRS score (p = 0.02; MD [95% CI]: -2.30[-4.31, -0.30]) and HAM-A score (p = 0.03; MD [95% CI]: -1.41[-2.70, -0.11]) on 15th day when compared to the Placebo group. Zuranolone was also significantly associated with a higher response rate (p = 0.0008; OR [95% CI]: 1.63[1.14, 2.35]) and higher remission rate (p = 0.03; OR [95% CI]: 1.65[1.05, 2.59]) when compared with the placebo. As for safety, Zuranolone was significantly associated with 1 or more TEAE (p = 0.006; RR [95% CI]: 1.14[1.04, 1.24]) but an insignificant association with side effects that lead to drug discontinuation (p = 0.70; RR [95% CI]: 1.18[0.51, 2.76]) and serious adverse events (p = 0.48; RR [95% CI]: 1.46 [0.52, 4.10]) when compared with placebo.
Conclusion: Zuranolone is an effective and safe drug for short course major depressive disorder monotherapy. It shows results in 14 days (compared to 2-4 weeks that SSRI's take) and has anti-anxiolytic effects as well. However, only 4 trials have been used for the analysis and the sample size was small. The trials reviewed also cannot determine the long-term effects of the drug. More trials are needed to determine long term effects.
Keywords: Zuranolone; antidepressants; depression; essential tremors; major depressive disorder; postpartum depression.
Copyright © 2024 Ahmad, Awan, Nadeem, Javed, Farooqi, Daniyal and Mumtaz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Evaluating the safety and efficacy of zuranolone in the management of major depressive disorder and postpartum depression, with or without concurrent insomnia: a rigorous systematic review and meta-analysis.Front Psychiatry. 2024 Jul 5;15:1425295. doi: 10.3389/fpsyt.2024.1425295. eCollection 2024. Front Psychiatry. 2024. PMID: 39035602 Free PMC article.
-
Effect of Zuranolone vs Placebo in Postpartum Depression: A Randomized Clinical Trial.JAMA Psychiatry. 2021 Sep 1;78(9):951-959. doi: 10.1001/jamapsychiatry.2021.1559. JAMA Psychiatry. 2021. PMID: 34190962 Free PMC article. Clinical Trial.
-
Efficacy and safety of zuranolone in the treatment of major depressive disorder: a meta-analysis.Front Neurosci. 2024 Jan 16;17:1332329. doi: 10.3389/fnins.2023.1332329. eCollection 2023. Front Neurosci. 2024. PMID: 38292895 Free PMC article.
-
Pharmacological interventions for treatment-resistant depression in adults.Cochrane Database Syst Rev. 2019 Dec 17;12(12):CD010557. doi: 10.1002/14651858.CD010557.pub2. Cochrane Database Syst Rev. 2019. PMID: 31846068 Free PMC article.
-
Zuranolone for the Treatment of Adults With Major Depressive Disorder: A Randomized, Placebo-Controlled Phase 3 Trial.Am J Psychiatry. 2023 Sep 1;180(9):676-684. doi: 10.1176/appi.ajp.20220459. Epub 2023 May 3. Am J Psychiatry. 2023. PMID: 37132201 Clinical Trial.
Cited by
-
Evaluating the safety and efficacy of zuranolone in the management of major depressive disorder and postpartum depression, with or without concurrent insomnia: a rigorous systematic review and meta-analysis.Front Psychiatry. 2024 Jul 5;15:1425295. doi: 10.3389/fpsyt.2024.1425295. eCollection 2024. Front Psychiatry. 2024. PMID: 39035602 Free PMC article.
-
New Agents in the Treatment of Psychiatric Disorders: What Innovations and in What Areas of Psychopathology?Pharmaceuticals (Basel). 2025 Apr 30;18(5):665. doi: 10.3390/ph18050665. Pharmaceuticals (Basel). 2025. PMID: 40430486 Free PMC article. Review.
References
-
- Althaus A. L., Ackley M. A., Belfort G. M., Gee S. M., Dai J., Nguyen D. P., et al. . (2020). Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology 181:108333. doi: 10.1016/j.neuropharm.2020.108333, PMID: - DOI - PMC - PubMed
-
- Arnaud A., Suthoff E., Stenson K., Werneburg B., Hodgkins P., Bonthapally V., et al. . (2021). Number needed to treat and number needed to harm analysis of the zuranolone phase 2 clinical trial results in major depressive disorder. J. Affect. Disord. 285, 112–119. doi: 10.1016/j.jad.2021.02.027, PMID: - DOI - PubMed
-
- Center for Behavioral Health Statistics . Results from the 2019 National Survey on drug use and health: detailed tables. Available at: https://www.samhsa.gov/data/
-
- Clayton A. H., Deligiannidis K. M., Kotecha M., Doherty J. (2022). Overview of the rapid antidepressant effects observed in the Zuranolone clinical development program [A164]. Obstet. Gynecol. 139, 47S–48S. doi: 10.1097/01.AOG.0000825932.04024.e0 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

