Benchmarking Guanidinium Organosulfonate Hydrogen-Bonded Frameworks for Structure Determination of Encapsulated Guests
- PMID: 38726044
- PMCID: PMC11077584
- DOI: 10.1021/acsmaterialslett.4c00400
Benchmarking Guanidinium Organosulfonate Hydrogen-Bonded Frameworks for Structure Determination of Encapsulated Guests
Abstract
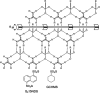
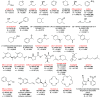
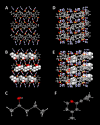
Single crystal X-ray diffraction (SCXRD) is arguably the most definitive method for molecular structure determination, but it is often challenged by compounds that are liquids or oils at room temperature or do not form crystals adequate for analysis. Our laboratory previously reported a simple, cost-effective, single-step crystallization method based on guanidinium organosulfonate (GS) hydrogen bonded frameworks for structure determination of a wide range of encapsulated guest molecules, including assignment of the absolute configuration of chiral centers. Herein, we expand on those results and report a head-to-head comparison of the GS method with adamantoid "molecular chaperones", which have been reported to be useful hosts for structure determination. Inclusion compounds limited to only two GS hosts are characterized by low R1 values and Flack parameters, infrequent disorder of the host and guest, and manageable disorder when it does exist. The structures of some target molecules that were not included or resolved using the adamantoid chaperones were successfully included and resolved by the GS hosts, and vice versa. Of the 32 guests attempted by the GS method, 31 inclusion compounds afforded successful guest structure solutions, a 97% success rate. The GS hosts and adamantoid chaperones are complementary with respect to guest inclusion, arguing that both should be employed in the arsenal of methods for structure determination. Furthermore, the low cost of organosulfonate host components promises an accessible route to molecular structure determination for a wide range of users.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Bragg W. H. The Reflection of X-Rays by Crystals. (II.). Proc. R. Soc. London Ser. Contain. Pap. Math. Phys. Character 1913, 89 (610), 246–248. 10.1098/rspa.1913.0082. - DOI
-
- Lonsdale K.; Whiddington R. The Structure of the Benzene Ring in C6 (CH3)6. Proc. R. Soc. London Ser. Contain. Pap. Math. Phys. Character 1929, 123 (792), 494–515. 10.1098/rspa.1929.0081. - DOI
-
- Bijvoet J. M.; Peerdeman A. F.; van Bommel A. J. Determination of the Absolute Configuration of Optically Active Compounds by Means of X-Rays. Nature 1951, 168 (4268), 271–272. 10.1038/168271a0. - DOI
LinkOut - more resources
Full Text Sources
