A programmable dual-targeting siRNA scaffold supports potent two-gene modulation in the central nervous system
- PMID: 38726879
- PMCID: PMC11194107
- DOI: 10.1093/nar/gkae368
A programmable dual-targeting siRNA scaffold supports potent two-gene modulation in the central nervous system
Abstract
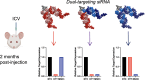
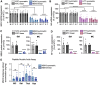
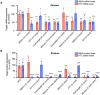
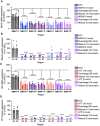
Divalent short-interfering RNA (siRNA) holds promise as a therapeutic approach allowing for the sequence-specific modulation of a target gene within the central nervous system (CNS). However, an siRNA modality capable of simultaneously modulating gene pairs would be invaluable for treating complex neurodegenerative disorders, where more than one pathway contributes to pathogenesis. Currently, the parameters and scaffold considerations for multi-targeting nucleic acid modalities in the CNS are undefined. Here, we propose a framework for designing unimolecular 'dual-targeting' divalent siRNAs capable of co-silencing two genes in the CNS. We systematically adjusted the original CNS-active divalent siRNA and identified that connecting two sense strands 3' and 5' through an intra-strand linker enabled a functional dual-targeting scaffold, greatly simplifying the synthetic process. Our findings demonstrate that the dual-targeting siRNA supports at least two months of maximal distribution and target silencing in the mouse CNS. The dual-targeting divalent siRNA is highly programmable, enabling simultaneous modulation of two different disease-relevant gene pairs (e.g. Huntington's disease: MSH3 and HTT; Alzheimer's disease: APOE and JAK1) with similar potency to a mixture of single-targeting divalent siRNAs against each gene. This work enhances the potential for CNS modulation of disease-related gene pairs using a unimolecular siRNA.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




Update of
-
A programmable dual-targeting di-valent siRNA scaffold supports potent multi-gene modulation in the central nervous system.bioRxiv [Preprint]. 2023 Dec 19:2023.12.19.572404. doi: 10.1101/2023.12.19.572404. bioRxiv. 2023. Update in: Nucleic Acids Res. 2024 Jun 24;52(11):6099-6113. doi: 10.1093/nar/gkae368. PMID: 38187561 Free PMC article. Updated. Preprint.
References
-
- Godinho B.M.D.C., Knox E.G., Hildebrand S., Gilbert J.W., Echeverria D., Kennedy Z., Haraszti R.A., Ferguson C.M., Coles A.H., Biscans A. et al. . PK-modifying anchors significantly alter clearance kinetics, tissue distribution, and efficacy of therapeutics siRNAs. Mol. Ther. Nucleic Acids. 2022; 29:116–132. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

