Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus
- PMID: 38726883
- PMCID: PMC11082932
- DOI: 10.1002/14651858.CD004661.pub4
Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus
Abstract
Background: Magnesium sulphate is a common therapy in perinatal care. Its benefits when given to women at risk of preterm birth for fetal neuroprotection (prevention of cerebral palsy for children) were shown in a 2009 Cochrane review. Internationally, use of magnesium sulphate for preterm cerebral palsy prevention is now recommended practice. As new randomised controlled trials (RCTs) and longer-term follow-up of prior RCTs have since been conducted, this review updates the previously published version.
Objectives: To assess the effectiveness and safety of magnesium sulphate as a fetal neuroprotective agent when given to women considered to be at risk of preterm birth.
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) on 17 March 2023, as well as reference lists of retrieved studies.
Selection criteria: We included RCTs and cluster-RCTs of women at risk of preterm birth that assessed prenatal magnesium sulphate for fetal neuroprotection compared with placebo or no treatment. All methods of administration (intravenous, intramuscular, and oral) were eligible. We did not include studies where magnesium sulphate was used with the primary aim of preterm labour tocolysis, or the prevention and/or treatment of eclampsia.
Data collection and analysis: Two review authors independently assessed RCTs for inclusion, extracted data, and assessed risk of bias and trustworthiness. Dichotomous data were presented as summary risk ratios (RR) with 95% confidence intervals (CI), and continuous data were presented as mean differences with 95% CI. We assessed the certainty of the evidence using the GRADE approach.
Main results: We included six RCTs (5917 women and their 6759 fetuses alive at randomisation). All RCTs were conducted in high-income countries. The RCTs compared magnesium sulphate with placebo in women at risk of preterm birth at less than 34 weeks' gestation; however, treatment regimens and inclusion/exclusion criteria varied. Though the RCTs were at an overall low risk of bias, the certainty of evidence ranged from high to very low, due to concerns regarding study limitations, imprecision, and inconsistency. Primary outcomes for infants/children: Up to two years' corrected age, magnesium sulphate compared with placebo reduced cerebral palsy (RR 0.71, 95% CI 0.57 to 0.89; 6 RCTs, 6107 children; number needed to treat for additional beneficial outcome (NNTB) 60, 95% CI 41 to 158) and death or cerebral palsy (RR 0.87, 95% CI 0.77 to 0.98; 6 RCTs, 6481 children; NNTB 56, 95% CI 32 to 363) (both high-certainty evidence). Magnesium sulphate probably resulted in little to no difference in death (fetal, neonatal, or later) (RR 0.96, 95% CI 0.82 to 1.13; 6 RCTs, 6759 children); major neurodevelopmental disability (RR 1.09, 95% CI 0.83 to 1.44; 1 RCT, 987 children); or death or major neurodevelopmental disability (RR 0.95, 95% CI 0.85 to 1.07; 3 RCTs, 4279 children) (all moderate-certainty evidence). At early school age, magnesium sulphate may have resulted in little to no difference in death (fetal, neonatal, or later) (RR 0.82, 95% CI 0.66 to 1.02; 2 RCTs, 1758 children); cerebral palsy (RR 0.99, 95% CI 0.69 to 1.41; 2 RCTs, 1038 children); death or cerebral palsy (RR 0.90, 95% CI 0.67 to 1.20; 1 RCT, 503 children); and death or major neurodevelopmental disability (RR 0.81, 95% CI 0.59 to 1.12; 1 RCT, 503 children) (all low-certainty evidence). Magnesium sulphate may also have resulted in little to no difference in major neurodevelopmental disability, but the evidence is very uncertain (average RR 0.92, 95% CI 0.53 to 1.62; 2 RCTs, 940 children; very low-certainty evidence). Secondary outcomes for infants/children: Magnesium sulphate probably reduced severe intraventricular haemorrhage (grade 3 or 4) (RR 0.76, 95% CI 0.60 to 0.98; 5 RCTs, 5885 infants; NNTB 92, 95% CI 55 to 1102; moderate-certainty evidence) and may have resulted in little to no difference in chronic lung disease/bronchopulmonary dysplasia (average RR 0.92, 95% CI 0.77 to 1.10; 5 RCTs, 6689 infants; low-certainty evidence). Primary outcomes for women: Magnesium sulphate may have resulted in little or no difference in severe maternal outcomes potentially related to treatment (death, cardiac arrest, respiratory arrest) (RR 0.32, 95% CI 0.01 to 7.92; 4 RCTs, 5300 women; low-certainty evidence). However, magnesium sulphate probably increased maternal adverse effects severe enough to stop treatment (average RR 3.21, 95% CI 1.88 to 5.48; 3 RCTs, 4736 women; moderate-certainty evidence). Secondary outcomes for women: Magnesium sulphate probably resulted in little to no difference in caesarean section (RR 0.96, 95% CI 0.91 to 1.02; 5 RCTs, 5861 women) and postpartum haemorrhage (RR 0.94, 95% CI 0.80 to 1.09; 2 RCTs, 2495 women) (both moderate-certainty evidence). Breastfeeding at hospital discharge and women's views of treatment were not reported.
Authors' conclusions: The currently available evidence indicates that magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus, compared with placebo, reduces cerebral palsy, and death or cerebral palsy, in children up to two years' corrected age, and probably reduces severe intraventricular haemorrhage for infants. Magnesium sulphate may result in little to no difference in outcomes in children at school age. While magnesium sulphate may result in little to no difference in severe maternal outcomes (death, cardiac arrest, respiratory arrest), it probably increases maternal adverse effects severe enough to stop treatment. Further research is needed on the longer-term benefits and harms for children, into adolescence and adulthood. Additional studies to determine variation in effects by characteristics of women treated and magnesium sulphate regimens used, along with the generalisability of findings to low- and middle-income countries, should be considered.
Copyright © 2024 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Emily Shepherd: former Editor for Cochrane Pregnancy and Childbirth and current Sign‐off Editor for Cochrane Central Editorial Service, but had no involvement in the editorial processing of this review.
Shona Goldsmith: senior research fellow with Cerebral Palsy Alliance, The University of Sydney.
Lex Doyle: investigator for an included randomised controlled trial (RCT) (Crowther 2003), and published opinions in medical journals relating to magnesium sulphate use in neuroprotection.
Philippa Middleton: investigator for an included RCT (Crowther 2023); former Editor for Cochrane Pregnancy and Childbirth and current Sign‐off Editor for Cochrane Central Editorial Service, but had no involvement in the editorial processing of this review; and Independent Contractor for National Health and Medical Research Council Stillbirth Centre for Research Excellence.
Stephane Marret: investigator for included RCT (Marret 2006), and works as a health professional in neonatology and neuropaediatrics, Rouen University Hospital, Rouen, France.
Dwight Rouse: investigator for an included RCT (Rouse 2008).
Peter Pryde: investigator for an included RCT (Mittendorf 2002); published opinions in medical journals relating to magnesium sulphate to reduce cerebral palsy; is a retired clinician; and maintains an adjunct faculty position at the University of Wisconsin School of Medicine and Public Health strictly for academic purposes.
Hanne Wolf: investigator for an included RCT (Wolf 2020), and works as a Gynaecologist and Obstetrician, University Hospital, Denmark.
Caroline Crowther: investigator for included RCTs (Crowther 2003; Crowther 2023).
Figures
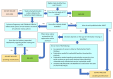
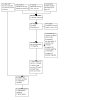
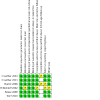



























































































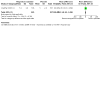






































Update of
-
Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD004661. doi: 10.1002/14651858.CD004661.pub3. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2024 May 10;5:CD004661. doi: 10.1002/14651858.CD004661.pub4. PMID: 19160238 Updated.
References
References to studies included in this review
Crowther 2003 {published and unpublished data}
-
- Crowther CA, Hiller JE, Doyle LW for the ACTOMgSO4 Collaborators Group. Does prenatal magnesium sulphate reduce the risk of mortality and cerebral palsy in infants born at less than 30 weeks' gestation? - The ACTOMgS04 trial. In: Perinatal Society of Australia and New Zealand 7th Annual Congress; 2003 March 9-12; Tasmania, Australia. 2003:A4.
-
- Crowther CA, Hiller JE, Doyle LW, Haslam RR for the Australasian Collaborative Trial of Magnesium Sulphate (ACTOMgSO4) Collaborative Group. Effect of magnesium sulfate given for neuroprotection before preterm birth. JAMA 2003;290(20):2669-76. - PubMed
-
- Crowther CA, Hiller JE, Doyle LW, Haslam RR. Is prenatal magnesium sulfate immediately prior to very preterm birth neuroprotective for babies? The ACTOMgSO4 trial: a randomized placebo-controlled trial. In: Pediatric Academic Societies Annual Meeting; 2003 May 3-6; Seattle, Washington, USA. 2003.
-
- Doyle LW, Anderson PJ, Haslam R, Lee KJ, Crowther C. School-age outcomes of very preterm infants after antenatal treatment with magnesium sulfate vs placebo. JAMA 2014;312(11):1105-13. - PubMed
-
- Paradisis M, Evans N, Osborn D, Kluckow M, ACTOMgSO4 Collaborators Group. The effect of antenatal magnesium sulphate on early systemic blood flow in very preterm infants. Pediatric Research 2004;55 Suppl:114.
Crowther 2023 {published data only}
-
- ACTRN12611000491965. MAGENTA - Magnesium Sulphate at 30 to 34 weeks' gestational age: neuroprotection trial [Does antenatal magnesium sulphate given to women at risk of imminent preterm birth (defined as planned or definitely expected in the next 24 hours) between 30 and 34 weeks' gestation reduce the risk of death or cerebral palsy in their children at 2 years corrected age? - a randomised controlled trial]. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12611000491965 (first received 11 May 2011).
Marret 2006 {published and unpublished data}
-
- Chollat C, Enser M, Houivet E, Provost D, Benichou J, Marpeau L, et al. School-age outcomes following a randomized controlled trial of magnesium sulfate for neuroprotection of preterm infants. Journal of Pediatrics 2014;165(2):398-400. - PubMed
-
- Chollat C, Marret S. Magnesium sulfate given before very-preterm birth to protect infant brain: the first long-term follow-up (PREMAG randomized trial). In: Pediatric Academic Societies Annual Meeting; 2013 May 4-7; Washington DC, USA. 2013.
-
- Marret S, Marpeau L, Astruc D, Cambonie G, Follet C, Benichou J. Prenatal magnesium sulfate (MgSO4) and follow up at two years of age in preterm infants: the randomised controlled PREMAG trial. In: Pediatric Academic Societies Annual Meeting; 2007 May 5-8; Toronto, Canada. 2007.
-
- Marret S, Marpeau L, Benichou J. Benefit of magnesium sulfate given before very preterm birth to protect infant brain. Pediatrics 2008;121(1):225-6. - PubMed
-
- Marret S, Marpeau L, Follet-Bouhamed C, Cambonie G, Astruc D, Delaporte B et al, for the PREMAG Group. Effect of magnesium sulphate on mortality and neurologic morbidity of the very preterm newborn with two-year neurological outcome: results of the prospective PREMAG trial [Effet du sulfate de magnésium sur la mortalité et la morbidité neurologique chez le prématuré de moins de 33 semaines, avec recul è deux ans: résultats de l'essai prospectif multicentrique contre placebo PREMAG]. Gynécologie Obstétrique & Fertilité 2008;36:278-88. - PubMed
Mittendorf 2002 {published data only}
-
- Mittendorf R, Bentz L, Borg M, Roizen N. Does exposure to antenatal magnesium sulfate prevent cerebral palsy? American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S20.
-
- Mittendorf R, Bentz L, Kohn J, Covert R. Use of antenatal magnesium sulfate does not seem to prevent intraventricular hemorrhage. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S34.
-
- Mittendorf R, Besinger R, Santillan M, Gianopoulos J. When used in circumstance of preterm labor, is there a paradoxical effect of varying exposures to magnesium sulfate (MGSO4) on the developing human brain? American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S65.
-
- Mittendorf R, Covert R, Boman J, Khoshnood B, Lee KS, Siegler M. Is tocolytic magnesium sulphate associated with increased total paediatric mortality? Lancet 1997;350(9090):1517-8. - PubMed
-
- Mittendorf R, Covert R, Elin R, Pryde P, Khoshnood B, Sun-Lee K. Umbilical cord serum ionized magnesium level and total pediatric mortality. Obstetrics & Gynecology 2001;98:75-8. - PubMed
Rouse 2008 {published data only}
-
- Brookfield KF, O'Malley K, Yeaton-Massey A, Butwick AJ. Does magnesium sulfate exposure attenuate the effect of steroids administered for fetal lung maturation? In: Society for Obstetric Anesthesia and Perinatology (SOAP) 48th Annual Meeting; 2016 May 18-22; Boston USA. 2016:SA-32.
-
- Costantine MM. Cord blood biomarkers and the risk of cerebral palsy or death. Reproductive Sciences 2010;17(3 Suppl 1):65A.
-
- Deihl TE, Simhan HN. Antenatal magnesium sulfate and ponderal index from birth to age 2 in preterm male and female infants. Reproductive Sciences 2017;24(Suppl 1):130A.
Wolf 2020 {published data only}
-
- Huusom LD, Brok J, Hegaard HK, Pryds O, Secher NJ. Does antenatal magnesium sulfate prevent cerebral palsy in preterm infants? the final trial? Acta Obstetricia et Gynecologica Scandinavica 2012;91(11):1346-7. - PubMed
-
- Larsen ML, Krebs L, Rackauskaite G, Hoei-Hansen CE, Greisen G. Re: antenatal magnesium sulphate for the prevention of cerebral palsy in infants born preterm: a double-blind, randomised, placebo-controlled, multi-centre trial: at which gestational ages should magnesium sulphate be given to women at risk of preterm birth? BJOG: an international journal of obstetrics and gynaecology 2020;127(10):1295-6. - PubMed
-
- NCT01492608. Magnesium Sulphate for Preterm Birth (MASP Study) [Administration of Antenatal Magnesium Sulphate for Prevention of Cerebral Palsy in Preterm Infants (MASP-STUDY)]. clinicaltrials.gov/show/nct01492608 (first received 15 December 2011).
-
- Wolf H, Huusom L, Pinborg A, Hegaard H. Administration of antenatal magnesium sulphate for prevention of cerebral palsy and death in preterm infants-a double blind randomised placebo controlled parallel group multicentre trial. Journal of Perinatal Medicine 2019;47(Suppl 1):eA167-8.
-
- Wolf HT, Brok J, Henriksen TB, Greisen G, Salvig JD, Pryds O, et al. Antenatal magnesium sulphate for the prevention of cerebral palsy in infants born preterm: a double-blind, randomised, placebo-controlled, multi-centre trial. BJOG: an international journal of obstetrics and gynaecology 2020;127(10):1217-25. - PubMed
References to studies excluded from this review
CTRI/2010/091/000578 {published data only}
-
- CTRI/2010/091/000578. CTRI/2010/091/000578 [A prospective observational study on effect of predelivery maternal factors on route of delivery in post cesarean women with intrauterine fetal death beyond 34 weeksâ?? gestation]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2010/091/000578 (first received 4 June 2010).
Dasgupta 2012 {published data only}
-
- Dasgupta S, Ghosh D, Seal SL, Kamilya G, Karmakar M, Saha D. Randomized controlled study comparing effect of magnesium sulfate with placebo on fetal umbilical artery and middle cerebral artery blood flow in mild preeclampsia at >= 34 weeks gestational age. Journal of Obstetrics and Gynaecology Research 2012;38(5):763-71. - PubMed
Gulczynska 2006 {published data only}
-
- Gulczynska EM, Gadzinowski J, Kesiak M, Sobolewska B, Grodzicka A, Zylinska L. The influence of erythropoietin (EPO) effect of maternal magnesium sulfate on red cells membrane lipid peroxidation - is there a correlation with clinical outcome in VLBWN? European Journal of Pediatrics 2006;165(Suppl 1):196-7.
Magpie 2006 {published data only}
NCT02591004 {published data only}
-
- NCT02591004. Calcium channel blockers compared to magnesium sulfate in fetal cerebral blood flow [Comparing fetal cerebral blood flow between magnesium sulfate & calcium channel blockers in patients with preterm labor; a randomized controlled trial]. clinicaltrials.gov/show/NCT02591004 (first received 19 October 2015).
Sayin 2010 {published data only}
-
- Sayin NC, Arda S, Varol FG, Sut N. The effects of ritodrine and magnesium sulfate on maternal and fetal Doppler blood flow patterns in women with preterm labor. European Journal of Obstetrics & Gynecology and Reproductive Biology 2010;152(1):50-4. - PubMed
References to studies awaiting assessment
Bachnas 2014 {published data only}
-
- Bachnas MA, Mose JC, Effendi JS, Andonotopo W. Influence of antenatal magnesium sulfate application on cord blood levels of brain-derived neurotrophic factor in premature infants. Journal of Perinatal Medicine 2014;42(1):129-34. - PubMed
Gupta 2021 {published data only}
-
- Gupta N, Garg R, Gupta A, Mishra S. Magnesium sulfate for fetal neuroprotection in women at risk of preterm birth: analysis of its effect on cerebral palsy [Magnesium sulfate for fetal neuroprotection in women at risk of preterm birth: Analysis of its effect on cerebral palsy]. Journal of SAFOG 2021;13(3):90-3.
Manoj 2017 {published data only}
-
- Manoj VC, Menon B, Sankar A, Anand M, Manikoth P. Randomized controlled trial of antenatal magnesium sulfate for short-term neuroprotection in premature neonates. Indian Journal of Child Health 2017;4(2):199-202.
Muhammed 2019 {published data only}
-
- Muhammed HO, Murad SJ. Effect of prenatal infusion of magnesium sulphate on neurological complication in preterm infant. International Journal of Pharmaceutical Research 2019;11(3):29-34.
Parashi 2017 {published data only}
-
- IRCT2016080729223N1. The survey of magnesium sulfate in prevention of intraventricular hemorrhage in premature infants [The survey of magnesium sulfate in prevention of intraventricular hemorrhage in premature infants: randomization clinical trial]. trialsearch.who.int/Trial2.aspx?TrialID=IRCT2016080729223N1 (first received 3 October 2016).
-
- Parashi S, Bordbar A, Mahmoodi Y, Jafari MR. The survey of magnesium sulfate in prevention of intraventricular hemorrhage in premature infants: a randomized clinical trial. Shiraz e Medical Journal 2017;18(11):e55094.
Petrov 2013 {published data only}
-
- Petrov V, Lupascu A, Etsco L, Pavlenco A. Maternal and new born hemodinamics after antenatal administration of magnesium sulfate (MGSO4), as a neuroprotective drug in preterm birth. Journal of Perinatal Medicine 2013;41:RU350.
-
- Petrov V, Lupascu A, Pavlenco A, Padure V, Burlacu A. The results of antenatal administration of magnesium sulfate (MGSO4), as a neuroprotective drug in preterm birth, at infants after one year follow up program. Journal of Perinatal Medicine 2013;41:RU369.
Sharma 2021 {published data only}
-
- Sharma K, Ranideepa A, Anamika B. To study the effect of antenatal magnesium sulphate for neuroprotection in preterm babies. International Journal of Advanced Research 2021;9(4):628-33.
Sheeba 2022 {published data only}
-
- Sheeba LM, Namboodiripad A, Varanattu MC. Efficacy of maternal magnesium sulfate administration on the neurodevelopmental outcome of preterm babies: a randomised controlled trial. Journal of Clinical and Diagnostic Research 2022;16(12):6-9.
References to ongoing studies
CTRI/2018/06/014386 {published data only}
-
- CTRI/2018/06/014386. Role of magnesium suplhate to mother in protecting brain of preterm babies [Antenatal magnesium sulphate for neuroprotection in preterm infants: An open label randomized control trial]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2018/06/014386 (first received 4 June 2018).
IRCT20120826010664N5 {published data only}
-
- IRCT20120826010664N5. Magnesium sulfate neuroprotection in preterm infants [Evaluation of neuroprotective effects of magnesium sulfate on preterm infants with a gestational age of 32-36 weeks]. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20120826010664N5 (first received 16 December 2019).
NCT02506894 {published data only}
-
- NCT02506894. Fetal middle cerebral artery doppler in preterm births receiving magnesium sulfate for neuroprotection. clinicaltrials.gov/show/NCT02506894 (first received 23 July 2015).
NCT05674565 {published data only}
-
- NCT05674565. Magnesium sulphate neuroprotective strategies for preterm deliveries [The neuroprotective impact of magnesium sulphate therapy for preterm deliveries. Loading dose alone strategy versus loading plus maintenance dose strategy]. clinicaltrials.gov/show/NCT05674565 (first received 6 January 2023).
Additional references
ACPR 2023
-
- ACPR Group. Australian Cerebral Palsy Register Report 2023. cpregister.com/wp-content/uploads/2023/01/2023-ACPR-Report.pdf (accessed 18 October 2023).
Bain 2013
Bekteshi 2023
-
- Bekteshi S, Monbaliu E, McIntyre S, Saloojee G, Hilberink SR, Tatishvili N, et al. Towards functional improvement of motor disorders associated with cerebral palsy. Lancet Neurology 2023;22:229-43. - PubMed
Carlisle 2017
-
- Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trial in anaesthetic and general medical journals. Anaesthesia 2017;72:944-52. - PubMed
Chollat 2019
-
- Chollat C, Sentilhes L, Marret S. Protection of brain development by antenatal magnesium sulphate for infants born preterm. Developmental Medicine & Child Neurology 2019;61(1):25-30. - PubMed
Conde‐Agudelo 2009
Costantine 2009
Crowther 2014
Crowther 2017
Duley 2010
Duley 2010a
Duley 2010b
Duley 2010c
Elliott 2016
Fishel 2022
-
- Fishel Bartal M, Sibai BM. Eclampsia in the 21st century. American Journal of Obstetrics and Gynecology 2022;226(2s):S1237-53. - PubMed
Galinsky 2020
Han 2013
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Jayaram 2019
-
- Jayaram PM, Mohan MK, Farid I, Lindow S. Antenatal magnesium sulfate for fetal neuroprotection: a critical appraisal and systematic review of clinical practice guidelines. Journal of Perinatal Medicine 2019;47(3):262-9. - PubMed
Kobayashi 2023
Korzeniewski 2018
-
- Korzeniewski SJ, Slaughter J, Lenski M, Haak P, Paneth N. The complex aetiology of cerebral palsy. Nature Reviews Neurology 2018;14(9):528-43. - PubMed
Kuban 1992
-
- Kuban K, Leviton A, Pagano M, Fenton T, Strasfeld R, Wolff M. Maternal toxemia is associated with reduced incidence of germinal matrix hemorrhage in premature babies. Journal of Child Neurology 1992;7:70-6. - PubMed
Lingam 2018
-
- Lingam I, Robertson NJ. Magnesium as a neuroprotective agent: A review of its use in the fetus, term infant with neonatal encephalopathy, and the adult stroke patient. Developmental Neuroscience 2018;40:1-12. - PubMed
Makrides 2014
McIntyre 2022
Nelson 1995
-
- Nelson KB, Grether JK. Can magnesium sulfate reduce the risk of cerebral palsy in very low birthweight infants? Pediatrics 1995;95:1-10. - PubMed
Novak 2017
RevMan 2024 [Computer program]
-
- Review Manager (RevMan). Version 7.2.0. The Cochrane Collaboration, 2024. Available at revman.cochrane.org.
Rosenbaum 2007
-
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Developmental Medicine & Child Neurology 2007;109:8-14. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strengthof recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shepherd 2017
-
- Shepherd E, Salam RA, Middleton P, Makrides M, McIntyre S, Badawi N, Crowther CA. Antenatal and intrapartum interventions for preventing cerebral palsy: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD012077. [DOI: 10.1002/14651858.CD012077.pub2] - DOI - PMC - PubMed
Shepherd 2018
-
- Shepherd E, Salam RA, Middleton P, Han S, Makrides M, McIntyre S, Badawi N, Crowther CA. Neonatal interventions for preventing cerebral palsy: an overview of Cochrane Systematic Reviews. Cochrane Database of Systematic Reviews 2018, Issue 6. Art. No: CD012409. [DOI: 10.1002/14651858.CD012409.pub2] - DOI - PMC - PubMed
Shepherd 2019
Shih 2018
-
- Shih STF, Tonmukayakul U, Imms C, Reddihough D, Graham HK, Cox L, Carter R. Economic evaluation and cost of interventions for cerebral palsy: a systematic review. Developmental Medicine & Child Neurology 2018;60(6):543-58. - PubMed
Spittle 2018
-
- Spittle AJ, Morgan C, Olsen JE, Novak I, Cheong JLY. Early diagnosis and treatment of cerebral palsy in children with a history of preterm birth. Clinics in Perinatology 2018;45:409-20. - PubMed
WHO 2015
-
- World Health Organization. WHO recommendations on interventions to improve preterm birth outcomes. www.who.int/publications/i/item/9789241508988;(accessed 18 October 2023).
Wolf 2012
-
- Wolf HT, Hegaard HK, Greisen G, Huusom L, Hedegaard M. Treatment with magnesium sulphate in pre-term birth: a systematic review and meta-analysis of observational studies. Journal of Obstetrics and Gynaecology 2012;32(2):135-40. - PubMed
Wolf 2020
-
- Wolf HT, Huusom LD, Henriksen TB, Heggard HK, Brok J, Pinborg A. Magnesium sulphate for fetal neuroprotection at imminent risk for preterm delivery: a systematic review with meta-analysis and trial sequential analysis. BJOG: an international journal of obstetrics & gynaecology 2020;127(10):1180-8. - PubMed
References to other published versions of this review
Crowther 2004
Doyle 2007
Doyle 2009
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

