JAK/STAT Inhibition Normalizes Lipid Composition in 3D Human Epidermal Equivalents Challenged with Th2 Cytokines
- PMID: 38727296
- PMCID: PMC11083560
- DOI: 10.3390/cells13090760
JAK/STAT Inhibition Normalizes Lipid Composition in 3D Human Epidermal Equivalents Challenged with Th2 Cytokines
Abstract
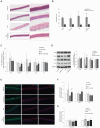
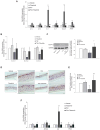
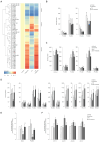
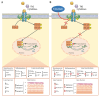
Derangement of the epidermal barrier lipids and dysregulated immune responses are key pathogenic features of atopic dermatitis (AD). The Th2-type cytokines interleukin IL-4 and IL-13 play a prominent role in AD by activating the Janus Kinase/Signal Transduction and Activator of Transcription (JAK/STAT) intracellular signaling axis. This study aimed to investigate the role of JAK/STAT in the lipid perturbations induced by Th2 signaling in 3D epidermal equivalents. Tofacitinib, a low-molecular-mass JAK inhibitor, was used to screen for JAK/STAT-mediated deregulation of lipid metabolism. Th2 cytokines decreased the expression of elongases 1, 3, and 4 and serine-palmitoyl-transferase and increased that of sphingolipid delta(4)-desaturase and carbonic anhydrase 2. Th2 cytokines inhibited the synthesis of palmitoleic acid and caused depletion of triglycerides, in association with altered phosphatidylcholine profiles and fatty acid (FA) metabolism. Overall, the ceramide profiles were minimally affected. Except for most sphingolipids and very-long-chain FAs, the effects of Th2 on lipid pathways were reversed by co-treatment with tofacitinib. An increase in the mRNA levels of CPT1A and ACAT1, reduced by tofacitinib, suggests that Th2 cytokines promote FA beta-oxidation. In conclusion, pharmacological inhibition of JAK/STAT activation prevents the lipid disruption caused by the halted homeostasis of FA metabolism.
Keywords: 3D skin model; JAK/STAT; Th2 cytokines; atopic dermatitis; skin lipidomics.
Conflict of interest statement
E.C. and G.C. were, respectively, the principal investigator (PI) and the Co-PI of this research project funded by Pfizer. A.C. received a research fellowship granted by Pfizer.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

