A Systematic Genotoxicity Assessment of a Suite of Metal Oxide Nanoparticles Reveals Their DNA Damaging and Clastogenic Potential
- PMID: 38727337
- PMCID: PMC11085103
- DOI: 10.3390/nano14090743
A Systematic Genotoxicity Assessment of a Suite of Metal Oxide Nanoparticles Reveals Their DNA Damaging and Clastogenic Potential
Abstract
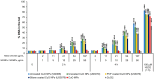
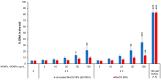
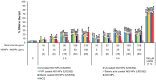
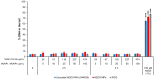
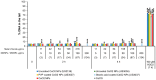
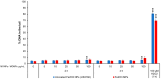
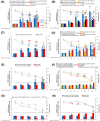
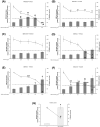
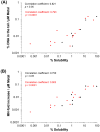
Metal oxide nanoparticles (MONP/s) induce DNA damage, which is influenced by their physicochemical properties. In this study, the high-throughput CometChip and micronucleus (MicroFlow) assays were used to investigate DNA and chromosomal damage in mouse lung epithelial cells induced by nano and bulk sizes of zinc oxide, copper oxide, manganese oxide, nickel oxide, aluminum oxide, cerium oxide, titanium dioxide, and iron oxide. Ionic forms of MONPs were also included. The study evaluated the impact of solubility, surface coating, and particle size on response. Correlation analysis showed that solubility in the cell culture medium was positively associated with response in both assays, with the nano form showing the same or higher response than larger particles. A subtle reduction in DNA damage response was observed post-exposure to some surface-coated MONPs. The observed difference in genotoxicity highlighted the mechanistic differences in the MONP-induced response, possibly influenced by both particle stability and chemical composition. The results highlight that combinations of properties influence response to MONPs and that solubility alone, while playing an important role, is not enough to explain the observed toxicity. The results have implications on the potential application of read-across strategies in support of human health risk assessment of MONPs.
Keywords: alveolar epithelial cells; comet assay; genotoxicity; lung toxicity; metal oxide nanoparticles; micronucleus; poorly soluble nanomaterials; soluble nanomaterials.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Health Canada Nanomaterials. [(accessed on 13 September 2023)]. Available online: https://www.canada.ca/en/health-canada/services/chemical-substances/nano....
-
- Health Canada Policy Statement on Health Canada’s Working Definition for Nanomaterial. [(accessed on 31 March 2024)]. Available online: https://www.canada.ca/en/health-canada/services/science-research/reports....
-
- Chavali M.S., Nikolova M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019;1:607. doi: 10.1007/s42452-019-0592-3. - DOI
-
- Piccinno F., Gottschalk F., Seeger S., Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012;14:1109. doi: 10.1007/s11051-012-1109-9. - DOI
-
- European Commission, Directorate-General for Environment . In: Support for 3rd Regulatory Review on Nanomaterials-Environmental Legislation. Broomfield M., Foss Hansen S., Pelsy F., editors. Publications Office of the European Union; Luxembourg: 2016. - DOI
LinkOut - more resources
Full Text Sources

