Rifaximin plus lactulose versus lactulose alone for reducing the risk of HE recurrence
- PMID: 38727685
- PMCID: PMC11093560
- DOI: 10.1097/HC9.0000000000000436
Rifaximin plus lactulose versus lactulose alone for reducing the risk of HE recurrence
Abstract
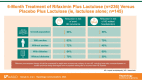
Background: The aim was to examine rifaximin plus lactulose efficacy in patients with cirrhosis at a risk of developing overt HE who were stratified by important baseline characteristics such as comorbid ascites or diabetes.
Methods: Pooled post hoc subgroup analysis of adults receiving rifaximin 550 mg twice daily plus lactulose or lactulose alone for 6 months in a phase 3 randomized, double-blind trial and a phase 4 open-label trial was conducted.
Results and conclusion: Rifaximin plus lactulose was more efficacious than lactulose alone for reducing the risk of overt HE recurrence and HE-related hospitalization in adults grouped by select baseline disease characteristics.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Arun J. Sanyal consults and received grants from Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Gilead, Intercept, Inventiva, Madrigal, Mallinckrodt, Merck, Novartis, Novo Nordisk, and Pfizer. He consults and owns stock in GENFIT and HemoShear. He consults for 89bio, Albireo, Alnylam, Amgen, AstraZeneca, Covance, Genentech, HistoIndex, Janssen, NGM Bio, PathAI, Poxel, ProSciento, Regeneron, Roche, Salix, Siemens, and Terns. He received grants from Fractyl. He owns stock in Durect, Exhalenz, Indalo, NorthSea, Rivus, and Tiziana. He receives royalties from Elsevier and UpToDate. Kris V. Kowdley consults, is on the speakers' bureau, and received grants from Gilead and Intercept. He consults and received grants from 89bio, CymaBay, GENFIT, Madrigal, Mirum, NGM, and Pfizer. He consults and owns stock in Inipharm. He consults for Enanta, HighTide, and Salix. He is on speakers' bureau for AbbVie. He received grants from Corcept, GlaxoSmithKline, Hanmi, Novo Nordisk, Pliant, Terns, and Viking. Nancy S. Reau consults for AbbVie, Gilead, Intercept, and Salix. She received grants from Eiger. Nikolaos T. Pyrsopoulos consults for Salix. Christopher Allen is employed by Salix. Zeev Heimanson is employed by Salix. Jasmohan S. Bajaj consults for Merz. He received grant from Cosmo, Grifols, Mallinckrodt, Salix, and Sequana Medical.
Figures


References
-
- D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J Hepatol. 2006;44:217–231. - PubMed
-
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. - PubMed
-
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. . Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. - PubMed
-
- Tapper EB, Parikh ND, Sengupta N, Mellinger J, Ratz D, Lok AS, et al. . A risk score to predict the development of hepatic encephalopathy in a population-based cohort of patients with cirrhosis. Hepatology. 2018;68:1498–1507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

