Recent Developments in Single-Entity Electrochemistry
- PMID: 38727715
- PMCID: PMC11112546
- DOI: 10.1021/acs.analchem.4c01406
Recent Developments in Single-Entity Electrochemistry
Conflict of interest statement
The authors declare no competing financial interest.
Figures

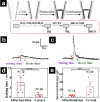

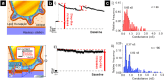

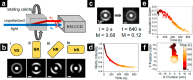

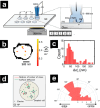
References
Publication types
LinkOut - more resources
Full Text Sources

