Knockdown of PHOX2B in the retrotrapezoid nucleus reduces the central CO2 chemoreflex in rats
- PMID: 38727716
- PMCID: PMC11087052
- DOI: 10.7554/eLife.94653
Knockdown of PHOX2B in the retrotrapezoid nucleus reduces the central CO2 chemoreflex in rats
Abstract
PHOX2B is a transcription factor essential for the development of different classes of neurons in the central and peripheral nervous system. Heterozygous mutations in the PHOX2B coding region are responsible for the occurrence of Congenital Central Hypoventilation Syndrome (CCHS), a rare neurological disorder characterised by inadequate chemosensitivity and life-threatening sleep-related hypoventilation. Animal studies suggest that chemoreflex defects are caused in part by the improper development or function of PHOX2B expressing neurons in the retrotrapezoid nucleus (RTN), a central hub for CO2 chemosensitivity. Although the function of PHOX2B in rodents during development is well established, its role in the adult respiratory network remains unknown. In this study, we investigated whether reduction in PHOX2B expression in chemosensitive neuromedin-B (NMB) expressing neurons in the RTN altered respiratory function. Four weeks following local RTN injection of a lentiviral vector expressing the short hairpin RNA (shRNA) targeting Phox2b mRNA, a reduction of PHOX2B expression was observed in Nmb neurons compared to both naive rats and rats injected with the non-target shRNA. PHOX2B knockdown did not affect breathing in room air or under hypoxia, but ventilation was significantly impaired during hypercapnia. PHOX2B knockdown did not alter Nmb expression but it was associated with reduced expression of both Task2 and Gpr4, two CO2/pH sensors in the RTN. We conclude that PHOX2B in the adult brain has an important role in CO2 chemoreception and reduced PHOX2B expression in CCHS beyond the developmental period may contribute to the impaired central chemoreflex function.
Keywords: PHOX2B; chemoreception; neuroscience; plethysmography; rat; retrotrapezoid nucleus; shRNA.
© 2024, Cardani, Janes et al.
Conflict of interest statement
SC, TJ, WB, SP No competing interests declared
Figures

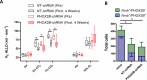
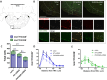
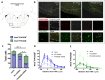
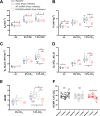
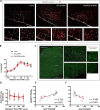
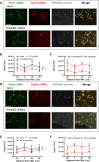
Update of
- doi: 10.1101/2023.12.13.571591
- doi: 10.7554/eLife.94653.1
- doi: 10.7554/eLife.94653.2
References
-
- Amiel J, Laudier B, Attié-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nature Genetics. 2003;33:459–461. doi: 10.1038/ng1130. - DOI - PubMed
-
- Bachetti T, Matera I, Borghini S, Di Duca M, Ravazzolo R, Ceccherini I. Distinct pathogenetic mechanisms for PHOX2B associated polyalanine expansions and frameshift mutations in congenital central hypoventilation syndrome. Human Molecular Genetics. 2005;14:1815–1824. doi: 10.1093/hmg/ddi188. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

