Normalization of Hemoglobin, Lactate Dehydrogenase, and Fatigue in Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with Pegcetacoplan
- PMID: 38727860
- PMCID: PMC11315842
- DOI: 10.1007/s40268-024-00463-9
Normalization of Hemoglobin, Lactate Dehydrogenase, and Fatigue in Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with Pegcetacoplan
Abstract
Background and objectives: We determined normalization rates for hemoglobin, lactate dehydrogenase (LDH), and fatigue in patients with paroxysmal nocturnal hemoglobinuria (PNH) treated with pegcetacoplan (PEG) in the PEGASUS (NCT03500549) and PRINCE (NCT04085601) phase III trials.
Methods: Enrolled patients had PNH and hemoglobin < 10.5 g/dL despite ≥ 3 months of eculizumab (ECU) [PEGASUS], or were complement component 5 (C5) inhibitor-naive, receiving supportive care only, with hemoglobin less than the lower limits of normal (LLN) [PRINCE]. Hematologic and fatigue normalization endpoints were hemoglobin greater than or equal to the LLN (females: 12 g/dL; males: 13.6 g/dL) in the absence of transfusion; LDH ≤226 U/L in the absence of transfusion; and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue ≥ 43.6, the general population norm. Safety was assessed by investigators using standardized terms and definitions for seriousness and severity.
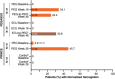
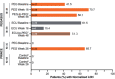
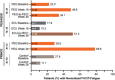
Results: Hemoglobin normalization occurred in 34.1% (14/41) of PEG-treated patients at Week 16 (randomized controlled period) in PEGASUS (vs. 0% [0/39] of ECU-treated patients) and in 45.7% (16/35) of PEG-treated patients at Week 26 in PRINCE (vs. 0% [0/18] of supportive care-treated patients). At Week 48 (open-label period) in PEGASUS, 24.4% of PEG-treated patients (PEG-to-PEG) and 30.8% of patients treated with ECU through Week 16 who switched to PEG through Week 48 (ECU-to-PEG) had hemoglobin normalization. Rates of LDH normalization in PEGASUS were 70.7% (PEG-treated patients) and 15.4% (ECU-treated patients) at Week 16, and 56.1% (PEG-to-PEG) and 51.3% (ECU-to-PEG) at Week 48. In PRINCE, 67.5% of PEG-treated patients at Week 26 had normalized LDH concentrations. Rates of FACIT-Fatigue score normalization in PEGASUS were 48.8% and 10.3% in PEG- and ECU-treated patients, respectively, at Week 16, and 34.1% and 51.3% in PEG-to-PEG- and ECU-to-PEG-treated patients, respectively, at Week 48. In PRINCE, 68.6% of PEG-treated patients and 11.1% of supportive care patients had FACIT-Fatigue score normalization at Week 26. PEG was safe and well tolerated. Injection site reactions, mostly mild, were the most common adverse event of special interest in PEG-treated patients in the PEGASUS randomized controlled period (36.6%) and in PRINCE (30.4%).
Conclusion: PEG is superior to ECU and supportive care in hemoglobin, LDH, and FACIT-Fatigue score normalization for patients with PNH and persistent anemia despite ≥3 months of treatment with ECU, and in C5 inhibitor-naive patients.
Clinical trial registration: The PEGASUS trial (NCT03500549) was registered on 18 August 2018, and the PRINCE trial (NCT04085601) was registered on 11 September 2019.
© 2024. The Author(s).
Conflict of interest statement
Brian P. Mulherin has no disclosures to declare. Michael Yeh is an employee of Apellis and holds stock options. Mohammed Al-Adhami was an employee of Apellis at the time of the study. David Dingli serves on advisory boards with compensation from Alexion (AstraZeneca), Apellis, Janssen, Novartis, and Sanofi; and receives research support from K-36 Therapeutics.
Figures
References
-
- Schrezenmeier H, Röth A, Araten DJ, et al. Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): updated analysis from the International PNH Registry. Ann Hematol. 2020;99(7):1505–14. 10.1007/s00277-020-04052-z. 10.1007/s00277-020-04052-z - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

