Multivariate Bayesian Optimization of CoO Nanoparticles for CO2 Hydrogenation Catalysis
- PMID: 38728108
- PMCID: PMC11117399
- DOI: 10.1021/jacs.4c03789
Multivariate Bayesian Optimization of CoO Nanoparticles for CO2 Hydrogenation Catalysis
Abstract
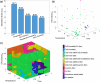
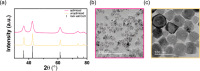
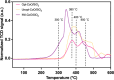
The hydrogenation of CO2 holds promise for transforming the production of renewable fuels and chemicals. However, the challenge lies in developing robust and selective catalysts for this process. Transition metal oxide catalysts, particularly cobalt oxide, have shown potential for CO2 hydrogenation, with performance heavily reliant on crystal phase and morphology. Achieving precise control over these catalyst attributes through colloidal nanoparticle synthesis could pave the way for catalyst and process advancement. Yet, navigating the complexities of colloidal nanoparticle syntheses, governed by numerous input variables, poses a significant challenge in systematically controlling resultant catalyst features. We present a multivariate Bayesian optimization, coupled with a data-driven classifier, to map the synthetic design space for colloidal CoO nanoparticles and simultaneously optimize them for multiple catalytically relevant features within a target crystalline phase. The optimized experimental conditions yielded small, phase-pure rock salt CoO nanoparticles of uniform size and shape. These optimized nanoparticles were then supported on SiO2 and assessed for thermocatalytic CO2 hydrogenation against larger, polydisperse CoO nanoparticles on SiO2 and a conventionally prepared catalyst. The optimized CoO/SiO2 catalyst consistently exhibited higher activity and CH4 selectivity (ca. 98%) across various pretreatment reduction temperatures as compared to the other catalysts. This remarkable performance was attributed to particle stability and consistent H* surface coverage, even after undergoing the highest temperature reduction, achieving a more stable catalytic species that resists sintering and carbon occlusion.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Wei X.; Johnson G.; Ye Y.; Cui M.; Yu S.-W.; Ran Y.; Cai J.; Liu Z.; Chen X.; Gao W.; Bean P. J. L.; Zhang W.; Zhao T. Y.; Perras F. A.; Crumlin E. J.; Zhang X.; Davis R. J.; Wu Z.; Zhang S. Surfactants Used in Colloidal Synthesis Modulate Ni Nanoparticle Surface Evolution for Selective CO2 Hydrogenation. J. Am. Chem. Soc. 2023, 145, 14298–14306. 10.1021/jacs.3c02739. - DOI - PubMed
-
- Iablokov V.; Beaumont S. K.; Alayoglu S.; Pushkarev V. V.; Specht C.; Gao J.; Alivisatos A. P.; Kruse N.; Somorjai G. A. Size-Controlled Model Co Nanoparticle Catalysts for CO2 Hydrogenation: Synthesis, Characterization, and Catalytic Reactions. Nano Lett. 2012, 12, 3091–3096. 10.1021/nl300973b. - DOI - PubMed
LinkOut - more resources
Full Text Sources

