Cetuximab plus FOLFOXIRI versus cetuximab plus FOLFOX as conversion regimen in RAS/BRAF wild-type patients with initially unresectable colorectal liver metastases (TRICE trial): A randomized controlled trial
- PMID: 38728364
- PMCID: PMC11086847
- DOI: 10.1371/journal.pmed.1004389
Cetuximab plus FOLFOXIRI versus cetuximab plus FOLFOX as conversion regimen in RAS/BRAF wild-type patients with initially unresectable colorectal liver metastases (TRICE trial): A randomized controlled trial
Abstract
Background: It remains unclear whether intensification of the chemotherapy backbone in tandem with an anti-EGFR can confer superior clinical outcomes in a cohort of RAS/BRAF wild-type colorectal cancer (CRC) patients with initially unresectable colorectal liver metastases (CRLM). To that end, we sought to comparatively evaluate the efficacy and safety of cetuximab plus FOLFOXIRI (triplet arm) versus cetuximab plus FOLFOX (doublet arm) as a conversion regimen (i.e., unresectable to resectable) in CRC patients with unresectable CRLM.
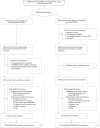
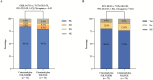
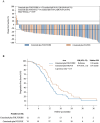
Methods and findings: This open-label, randomized clinical trial was conducted from April 2018 to December 2022 in 7 medical centers across China, enrolling 146 RAS/BRAF wild-type CRC patients with initially unresectable CRLM. A stratified blocked randomization method was utilized to assign patients (1:1) to either the cetuximab plus FOLFOXIRI (n = 72) or cetuximab plus FOLFOX (n = 74) treatment arms. Stratification factors were tumor location (left versus right) and resectability (technically unresectable versus ≥5 metastases). The primary outcome was the objective response rate (ORR). Secondary outcomes included the median depth of tumor response (DpR), early tumor shrinkage (ETS), R0 resection rate, progression-free survival (PFS), overall survival (not mature at the time of analysis), and safety profile. Radiological tumor evaluations were conducted by radiologists blinded to the group allocation. Primary efficacy analyses were conducted based on the intention-to-treat population, while safety analyses were performed on patients who received at least 1 line of chemotherapy. A total of 14 patients (9.6%) were lost to follow-up (9 in the doublet arm and 5 in the triplet arm). The ORR was comparable following adjustment for stratification factors, with 84.7% versus 79.7% in the triplet and doublet arms, respectively (odds ratio [OR] 0.70; 95% confidence intervals [CI] [0.30, 1.67], Chi-square p = 0.42). Moreover, the ETS rate showed no significant difference between the triplet and doublet arms (80.6% (58/72) versus 77.0% (57/74), OR 0.82, 95% CI [0.37, 1.83], Chi-square p = 0.63). Although median DpR was higher in the triplet therapy group (59.6%, interquartile range [IQR], [50.0, 69.7] versus 55.0%, IQR [42.8, 63.8], Mann-Whitney p = 0.039), the R0/R1 resection rate with or without radiofrequency ablation/stereotactic body radiation therapy was comparable with 54.2% (39/72) of patients in the triplet arm versus 52.7% (39/74) in the doublet arm. At a median follow-up of 26.2 months (IQR [12.8, 40.5]), the median PFS was 11.8 months in the triplet arm versus 13.4 months in the doublet arm (hazard ratio [HR] 0.74, 95% CI [0.50, 1.11], Log-rank p = 0.14). Grade ≥ 3 events were reported in 47.2% (35/74) of patients in the doublet arm and 55.9% (38/68) of patients in the triplet arm. The triplet arm was associated with a higher incidence of grade ≥ 3 neutropenia (44.1% versus 27.0%, p = 0.03) and diarrhea (5.9% versus 0%, p = 0.03). The primary limitations of the study encompass the inherent bias in subjective surgical decisions regarding resection feasibility, as well as the lack of a centralized assessment for ORR and resection.
Conclusions: The combination of cetuximab with FOLFOXIRI did not significantly improve ORR compared to cetuximab plus FOLFOX. Despite achieving an enhanced DpR, this improvement did not translate into improved R0 resection rates or PFS. Moreover, the triplet arm was associated with an increase in treatment-related toxicity.
Trial registration: ClinicalTrials.gov Identifier: NCT03493048.
Copyright: © 2024 Wang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al.. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–57; discussion 57–8. doi: 10.1097/01.sla.0000141198.92114.f6 ; PubMed Central PMCID: PMC1356466. - DOI - PMC - PubMed
-
- Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al.. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818–25; discussion 25–7. doi: 10.1097/01.sla.0000128305.90650.71 ; PubMed Central PMCID: PMC1356290. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

