How I use next-generation sequencing-MRD to plan approach and prevent relapse after HCT for children and adults with ALL
- PMID: 38728375
- PMCID: PMC11302453
- DOI: 10.1182/blood.2023023699
How I use next-generation sequencing-MRD to plan approach and prevent relapse after HCT for children and adults with ALL
Abstract
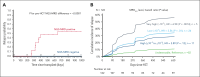
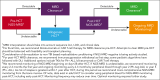
Measurable residual disease (MRD) evaluation by multiparameter flow cytometry (MFC) or quantitative polymerase chain reaction methods is an established standard of care for assessing risk of relapse before or after hematopoietic cell transplantation (HCT) for acute lymphoblastic leukemia (ALL). Next-generation sequencing (NGS)-MRD has emerged as a highly effective approach that allows for the detection of lymphoblasts at a level of <1 in 106 nucleated cells, increasing sensitivity of ALL detection by 2 to 3 logs. Early studies have shown superior results compared with MFC and suggest that NGS-MRD may allow for the determination of patients in whom reduced toxicity transplant preparative approaches could be deployed without sacrificing outcomes. Many centers/study groups have implemented immune modulation approaches based on MRD measurements that have resulted in improved outcomes. Challenges remain with NGS-MRD, because it is not commercially available in many countries, and interpretation of results can be complex. Through patient case review, discussion of relevant studies, and detailed expert opinion, we share our approach to NGS-MRD testing before and after HCT in pediatric and adult ALL. Improved pre-HCT risk classification and post-HCT monitoring for relapse in bone marrow and less invasive peripheral blood monitoring by NGS-MRD may lead to alternative approaches to prevent relapse in patients undergoing this challenging procedure.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: L.M. reports advisory board fees from Kite, Autolus, Vor, and Cargo; research support from Jasper, Kite, Adaptive Biotechnologies, Bristol Myers Squibb, Astellas, Wugen, and Vor; honoraria from Kite; and consultancy from Astellas. M.A.P. reports advisory board fees from Pfizer, Cargo, Novartis, GentiBio, Bluebird, and Vertex; steering committee membership for Novartis and Autolus; and research support from Miltenyi and Adaptive Biotechnologies. The remaining authors declare no competing financial interests.
Figures


References
-
- US Food and Drug Administration FDA authorizes first next generation sequencing-based test to detect very low levels of remaining cancer cells in patients with acute lymphoblastic leukemia or multiple myeloma. https://www.fda.gov/news-events/press-announcements/fda-authorizes-first... Released 18 September 2018.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

