Antigen escape as a shared mechanism of resistance to BCMA-directed therapies in multiple myeloma
- PMID: 38728378
- PMCID: PMC11302451
- DOI: 10.1182/blood.2023023557
Antigen escape as a shared mechanism of resistance to BCMA-directed therapies in multiple myeloma
Abstract
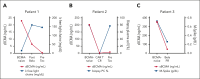
B-cell maturation antigen (BCMA)-targeting therapeutics have dramatically improved outcomes in relapsed/refractory multiple myeloma (RRMM). However, whether the mechanisms of resistance between these therapies are shared and how the identification of such mechanisms before therapy initiation could refine clinical decision-making remains undefined. We analyzed outcomes for 72 RRMM patients treated with teclistamab, a CD3 × BCMA bispecific antibody, 42% (30/72) of whom had prior BCMA-directed therapy exposure. Malignant plasma cell BCMA expression was present in all BCMA therapy-naïve patients. Prior therapy-mediated loss of plasma cell BCMA expression before teclistamab treatment, measured by immunohistochemistry, was observed in 3 patients, none of whom responded to teclistamab, and 1 of whom also did not respond to ciltacabtagene autoleucel. Whole exome sequencing of tumor DNA from 1 patient revealed biallelic loss of TNFRSF17 following treatment with belantamab mafodotin. Low-to-undetectable peripheral blood soluble BCMA levels correlated with the absence of BCMA expression by bone marrow plasma cells. Thus, although rare, loss of BCMA expression following TNFRSF17 gene deletions can occur following any BCMA-directed therapy and prevents response to subsequent anti-BCMA-directed treatments, underscoring the importance of verifying the presence of a target antigen.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: T.S. reports receiving honoraria from Roche-Genentech. M.Z. reports receiving advisory or consulting fees from Leica Biosystems. M.H. reports research funding from Amgen, Daiichi Sankyo, GlaxoSmithKline (GSK); and has received honoraria for consultancy/participated in advisory boards for Curio Science LLC, Intellisphere LLC, Bristol Myers Squibb (BMS), and GSK. S.M. received consulting fees from Evicore, Optum, BioAscend, Janssen Oncology, BMS, AbbVie, HMP Education, and Legend Biotech; and received honoraria from OncLive, Physician Education Resource, MJH Life Sciences, and Plexus Communications. Memorial Sloan Kettering Cancer Center receives research funding from the National Cancer Institute, Janssen Oncology, BMS, Allogene Therapeutics, Fate Therapeutics, Caribou Therapeutics, and Takeda Oncology for conducting research. C.R.T. reports research funding from Janssen and Takeda and personal fees from Physician Educations Resource and MJH Life Sciences; and has participated in advisory boards for Janssen and Sanofi, outside of the submitted work. N.K. reports research funding through Amgen, Janssen, Epizyme, AbbVie; consults for Clinical Care Options, OncLive, and Intellisphere Remedy Health; and participated in advisory board for Janssen and MedImmune. A.M.L. reports receiving grants from Novartis, during the conduct of the study; grants from BMS; personal fees from Trillium Therapeutics; grants, personal fees, and nonfinancial support from Pfizer; grants and personal fees from Janssen, outside the submitted work; and also has a patent (number US20150037346A1) with royalties paid. H.H. reports grants from Celgene, Takeda, and Janssen, outside the submitted work. U.S. reports research support from Celgene/BMS and Janssen; personal fees from MashUp MD, Janssen Biotech, Sanofi, BMS, MJH Life Sciences, Intellisphere, Phillips Gilmore Oncology Communications, i3 Health, and RedMedEd. H.J.L. has served as a paid consultant for Takeda, Genzyme, Janssen, Karyopharm, Pfizer, Celgene, Caelum Biosciences, and has received research support from Takeda. M.S. served as a paid consultant for McKinsey & Company, Angiocrine Bioscience, Inc, and Omeros Corporation; received research funding from Angiocrine Bioscience, Inc, Omeros Corporation, and Amgen, Inc; served on ad hoc advisory boards for Kite, a Gilead company; and received honoraria from i3 Health, Medscape, and CancerNetwork for CME-related activity. G.L.S. receives research funding from Janssen, Amgen, BMS, Beyond Spring; serves on the data safety monitoring board (DSMB) for ArcellX; and receives research funding to the institution from Janssen, Amgen, BMS, Beyond Spring, and GPCR. O.B.L. reports serving on advisory board for MorphoSys, Kite, Daiichi Sankyo Inc, and Incyte and has served as a paid consultant for Incyte. S.G. reports personal fees and advisory role (scientific advisory board) from Actinium, Celgene, BMS, Sanofi, Amgen, Pfizer, GSK, Jazz, Janssen, Omeros, Takeda, and Kite, outside the submitted work. K.M. reports funding from Sebia, Binding site, and Siemens. S.Z.U. reports grants and personal fees from AbbVie, 404 Amgen, BMS, Celgene, GSK, Janssen, Merck, Mundipharma, Oncopeptides, 405 Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, and Takeda. D.J.C. receives research funding from Genentech. The remaining authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

